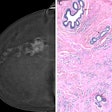
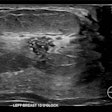
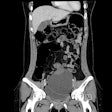
A mammography-based AI model showed good and generalizable performance across European screening populations, according to study results delivered on 7 December at the San Antonio Breast Cancer Symposium in Texas.
Presenter Mikael Eriksson, PhD, from Karolinska Institute in Stockholm, Sweden, shared findings that showed the performance of his team’s AI model, which captured nearly one in three clinically relevant stage II and higher breast cancers in high-risk women with a negative mammogram.
“An image-derived AI model is feasible for personalized screening to improve screening outcomes,” Eriksson said.
Previous research has shown the ability of image-based AI models to discriminate between populations when it comes to assessing breast cancer risk based on family history and lifestyle factors. Eriksson previously led a study showing that a model he and colleagues developed outperformed the Tyrer-Cuzick version 8 model for both short-term and long-term risk assessment of breast cancer.
However, Eriksson noted that there is little data available on the generalizability and clinical feasibility of these AI models across different screening settings.
For their study, Eriksson and colleagues tested their clinically used image-derived, AI-based risk model in multiple European breast cancer screening populations. From full-field digital mammograms, the AI extracted mammographic features including density, microcalcifications, masses, and left-right breast asymmetries for risk assessment.
The model was tested on four European mammographic screening populations in three countries screened between 2009 and 2020 for women ages 45 to 69. In total, the team included 739 women with incident breast cancer together with 7,812 matched controls. It assessed breast cancer occurrence after two years of follow-up.
The model achieved an area under the curve of 0.72, with a range of 0.71 to 0.74, for breast cancers developed in all screening populations in the study.
The team also reported that 4.6% of women were classified as high-risk following guidelines by the National Institute for Health and Care Excellence (NICE). For these women, breast cancers were “more likely” diagnosed after two years follow-up, Eriksson said. The risk ratio for these women was high, at 6.7 (with 1 as reference). In comparison, the model classified 71% of women in the study as general risk.
Eriksson added that similar risk-ratio values were reported across differing mammographic densities. Finally, the team found that in high-risk women, 22% of the two-year future cancers were diagnosed, 29% of which were stage II and higher cancers (p < 0.01).
“If [high-risk women] had supplemental screening, the cancers would have the potential to be found earlier,” Eriksson said.
Eriksson called for randomized clinical trials using such models to study their potential for personalized screening.
The study was also published on 6 December in The Lancet.