The integration of ultrasound-guided thermoprotection techniques, whether used alone or in combination, is an effective strategy for safeguarding vital structures and significantly enhancing safety in cases of hepatocellular carcinoma (HCC), Australian researchers have reported.
"When meticulously applied in clinical practice, these methods directly improve patient outcomes and reduce procedural risks," noted Dr. Bruno Di Muzio, a consultant radiologist at Alfred Hospital in Melbourne and adjunct clinical fellow at Monash University, and colleagues.
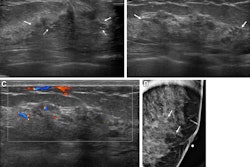
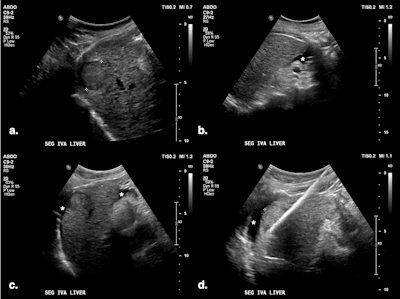
 (A) Targeted HCC in segment VIII. (B) Microwave ablation in progress, with artificial ascites (*) separating the liver from the diaphragm. (C) Oblique image compared with the previous one, showing further liver displacement from the diaphragm through mechanical leveraging and illustrating continuous monitoring of the ablation zone. All figures courtesy of Dr. Bruno Di Muzio and Department of Radiology, Alfred Health, Melbourne, and presented at RANZCR's 2024 ASM.
(A) Targeted HCC in segment VIII. (B) Microwave ablation in progress, with artificial ascites (*) separating the liver from the diaphragm. (C) Oblique image compared with the previous one, showing further liver displacement from the diaphragm through mechanical leveraging and illustrating continuous monitoring of the ablation zone. All figures courtesy of Dr. Bruno Di Muzio and Department of Radiology, Alfred Health, Melbourne, and presented at RANZCR's 2024 ASM.
In Australia, the incidence of HCC has been increasing more rapidly than that of other cancers. It often arises in the context of chronic liver disease, with the dominant etiologies being cirrhosis, chronic hepatitis B infection, alcohol misuse, and metabolic dysfunction-associated steatohepatitis (MASH), they explained in an e-poster at the annual scientific meeting (ASM) of the Royal Australian and New Zealand College of Radiologists (RANZCR), held recently in Perth.
"Besides the well-established use in HCC, there has been a growing use of thermal ablation for the treatment of oligometastatic disease," the authors wrote. "Studies have shown that, compared to surgery, microwave ablation can yield comparable results in treating liver metastases from colorectal cancer."
Percutaneous approaches for thermal ablation are commonly guided by ultrasound, CT, or a combination of both, but peripheral liver lesions located near vital organs such as the bowel, stomach, kidneys, gallbladder, diaphragm, and heart -- especially those within 10 mm -- present a challenge due to the risk of collateral thermal injury. The Melbourne group uses several thermo-protective techniques to safeguard these organs and minimize complications.
Artificial ascites (hydro dissection)
Artificial ascites involves creating a fluid layer around the liver near the treated area to protect adjacent organs from thermal injury. For hepatic dome tumors, this technique also reduces postprocedure pain caused by diaphragmatic irritation and may create an acoustic window enabling access with ultrasound guidance, the authors pointed out.
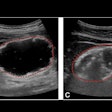
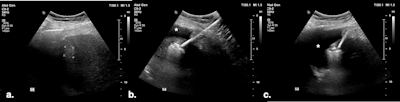
 (A) Targeted HCC in segment IV-A near the diaphragm. (B) Artificial ascites (*) created using a 6.3 Fr Dawson-Mueller catheter inserted along the inferior margin of the left hepatic lobe. (C) Satisfactory distribution of ascites beneath the diaphragm. (D) Microwave antenna inserted at the target, with mechanical leveraging performed to further accentuate the gap between the liver and diaphragm.
(A) Targeted HCC in segment IV-A near the diaphragm. (B) Artificial ascites (*) created using a 6.3 Fr Dawson-Mueller catheter inserted along the inferior margin of the left hepatic lobe. (C) Satisfactory distribution of ascites beneath the diaphragm. (D) Microwave antenna inserted at the target, with mechanical leveraging performed to further accentuate the gap between the liver and diaphragm.
Ultrasound-guided puncture at the peritoneal interface uses the liver, gallbladder, or stomach walls as a reference, and a catheter is deployed via a direct method or the Seldinger technique.
Di Muzio and colleagues prefer the 6.3 Fr Dawson-Mueller catheter for creating artificial ascites, but alternatives include a 14-20 G Chiba needle, One-Step Centesis Catheter, or a 5 Fr vascular catheter/sheath. They use a 1 L saline bag with a pressure cuff to expedite the infusion process, and the fluid distribution and liver displacement are monitored with ultrasound. There is no consensus on how thick the layer of fluid separating the structures should be, with the literature reporting between 5 and 10 mm.
Previous liver resection or other abdominal surgeries may result in adhesions precluding the fluid dissipation to the area of interest, they added.
Artificial pleural effusion
In this procedure, fluid is introduced into the pleural cavity to create an acoustic window, allowing ultrasound-guided access to tumors located in the hepatic dome region. In this context, it does not serve to protect the diaphragm from collateral thermal injury, the authors noted.
Ultrasound-guided puncture at the costophrenic angle involves using an 18-gauge BD Angiocath needle and observing visceral pleural movements. Once the needle enters the pleural cavity, confirmed by the injection of 10 mL of saline, the outer sheath is advanced to avoid lung injury. The Seldinger technique may be then applied to deploy a larger catheter (e.g., 6.3 Fr Dawson-Mueller).
A 1 L saline bag is used with a pressure cuff to expedite the infusion process, and the fluid distribution is monitored with ultrasound; the volume infused may vary by 500 mL to 1,500 mL. After the procedure, depending on the amount of fluid infused, pleural effusion can be drained through the sheath or catheter.
Previous pleurodesis and chronic pleural disease may limit control over fluid distribution, they wrote.
Mechanical liver displacement (leverage)
This procedure uses a microwave antenna to displace the liver away from at-risk structures, usually in combination with artificial ascites, enhancing the protection of adjacent viscera against thermal injury. It can be useful in patients with adhesions.
"Mechanical displacement technique involves tilting the microwave needle handle to shift the liver away, thereby creating a wider gap between the ablation zone and organs at risk of collateral thermal injury," the authors stated. "When treating cirrhotic livers, which are typically stiff and smaller in volume, leveraging techniques can achieve significant displacement."
Nontouch technique
Although primarily used for subcapsular tumors to prevent capsular breach, the nontouch technique also serves as a thermoprotection measure by ensuring that the heating core is positioned further away from at-risk viscera. The ablation antenna is inserted tangentially around the tumor rather than directly into it, without penetrating it. This method creates a larger, more uniform ablation zone that includes the subcapsular tumor and the surrounding tissue, but it keeps the actual ablation activity away from the liver surface, they explained.
Intermittent direct monitoring of ablation progress to control extension of the ablation zone and ensures procedural accuracy. Regular ultrasound checks should be carried out during ablation to assess distribution of the ablation zone, permitting timely intervention or cessation if necessary to prevent thermal injury.
The Melbourne team is close to completing a comprehensive 20-year review of its experience with HCC thermal ablation, which has been heavily guided by ultrasound. It will be submitted soon for publication.
You can read the full e-poster on the EPOS section for the RANZCR ASM 2024 via the ESR's website. The co-authors were Dr. Wa Cheung, consultant radiologist at Alfred Hospital, Melbourne, and Dr. Ilycia Wutami, a resident at Alfred Hospital.