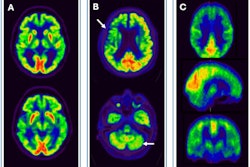
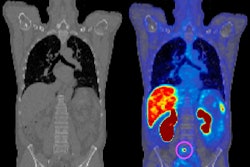
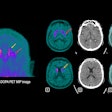
Telix Pharmaceuticals’ prostate cancer PET imaging kit Illuccix has received marketing authorization in France and Finland.
Illuccix, after radiolabeling with gallium-68, is indicated in the two countries for the detection of prostate-specific membrane antigen (PSMA)-positive lesions with PET in adults with prostate cancer in the following clinical settings:
- Primary staging of patients with high-risk prostate cancer prior to primary curative therapy.
- Suspected recurrent prostate cancer in patients with increasing levels of serum prostate-specific antigen after primary curative therapy.
- Identification of patients with PSMA-positive progressive metastatic castration-resistant prostate cancer for whom PSMA-targeted therapy is indicated.
In France, Telix will partner with IRE ELiT S.A. (the radiopharmaceutical subsidiary of IRE Group) for the marketing and promotion of Illuccix to healthcare professionals; in Finland, Illuccix will be made available through Telix's distribution partner for the Nordic region, WIIK Pharma.
The approvals come as demand for PSMA-PET continues to grow across Europe, Telix noted.