The University Hospital Münster, Helmholtz Munich, and ITM Isotope Technologies Munich have announced that the first patient has been dosed in a trial with ITM’s radiopharmaceutical drug candidate, ITM-31, for use in glioblastoma patients.
The trial is sponsored by the University Hospital Münster, conducted in hospitals in Münster, Essen, Cologne, and Würzburg, and supported by Helmholtz Munich and ITM.
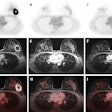
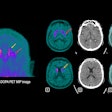
ITM-31 is a carbonic anhydrase XII-specific antibody Fab fragment developed by Helmholtz Munich and coupled with ITM's medical radioisotope, non-carrier-added lutetium-177 (Lu-177).
The dose-escalation study is enrolling up to 15 patients and will evaluate the impact of ITM-31 on glioblastoma patients by analyzing the tolerability and safety of ITM-31 while evaluating the best possible patient dose for future studies.