The use of somatostatin receptor PET imaging and therapy in meningiomas is on the rise, and new guidelines on their use can help pave the way to improved outcomes for patients, European experts have noted.
“The application of radiolabeled [somatostatin receptor] ligands is widely considered to have high potential for improved meningioma management,” wrote lead author Dr. Nathalie Albert, of the Ludwig Maximilian University of Munich, and colleagues. The article was published on 10 October in the Journal of Nuclear Medicine.
Albert and colleagues from the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, the European Association of Neuro-Oncology, and the PET task force of the Response Assessment in Neuro-Oncology Working Group recently developed practice guidelines for the approach, which were published in June in the European Journal of Nuclear Medicine and Molecular Imaging.
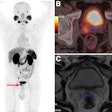
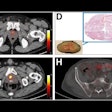
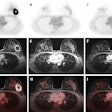
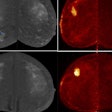
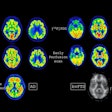
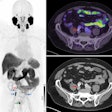
Meningiomas are the most common primary adult intracranial tumors and most meningiomas show high expression of somatostatin receptors (SSTRs). These SSTRs can be targeted both with gallium-68 (Ga-68) imaging agents and therapeutic lutetium-177 (Lu-177) radiopharmaceuticals using a so-called theranostics approach.
In the U.S., for instance, Ga-68 DOTATATE and copper-64 DOTATATE are two readily available SSRT PET radiotracers, with a variable but overall increasing degree of insurance coverage, the authors noted. In addition, a recent study demonstrated the cost-effectiveness of Ga-68 DOTATATE PET/MRI in the management of meningiomas in a U.S. healthcare setting, they wrote.
In addition, importantly, the first randomized trial evaluating Lu-177 DOTATATE for recurrent meningioma is scheduled to start enrolling patients in the first quarter of 2025 and will for the first time provide controlled data on radiopharmaceutical therapy for meningiomas.
“Overall, these developments have led to an increase in the use of SSTR-directed PET imaging and therapy in routine clinical practice and its integration into clinical trials,” the authors wrote.
The guidelines provide detailed information on a wide range of topics, including equipment and tracer types, patient procedures, criteria for image analysis, dosing and administration regimens of radiopharmaceuticals, recommended comedications, dosimetry, monitoring and follow-up schedules, side effect prevention and management, and quality control measures.
Moreover, the guidelines will further facilitate health insurance reimbursement for SSTR-targeted PET in meningioma, thereby increasing patient access to care and improving clinical outcomes, the group added.
“Globally, this first edition of the guidelines and procedure standards will enable harmonization and standardization and is therefore an important contribution toward evidence-based and safe application of SSTR-targeted PET imaging and radiopharmaceutical therapy in patients with meningioma around the world,” Albert and colleagues concluded.
The full article is available here.