The application of advanced imaging techniques in brain cancer treatment is evolving fast, particularly the quantitative evaluation of the use of radiotracers in cases of glioblastoma, scientists have reported.
The F-18 fluoroethyl tyrosine (FET)-PET in glioblastoma (FIG) study is an active Australian prospective, multicenter trial evaluating FET-PET for adult glioblastoma management. Participant imaging consists of pre-chemoradiotherapy (FET-PET1), one month after chemoradiotherapy (FET-PET2), and at suspected progression (FET-PET3), according to Nathaniel Barry, a medical physics PhD student at the University of Western Australia in Perth, trial co-chairs Dr. Eng-Siew Koh and Dr. Andrew Scott, PhD, and colleagues.
"Prior to site activation, radiation oncologists and sites participated in a mandatory credentialing program," they noted in a poster at the annual scientific meeting (ASM) of the Royal Australian and New Zealand College of Radiologists (RANZCR), held recently in Brisbane.
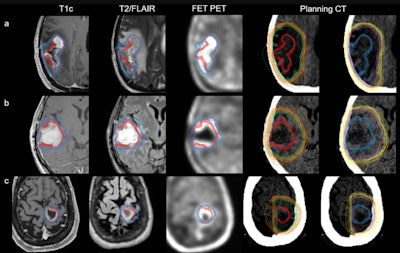 An overview of all radiation oncology target volume delineations on axial imaging. Each row represents the benchmarking cases: FET-PET11 case 1 (a), FET-PET1 case 2 (b), and FET-PET1 case 3 (c). Shown here are standard gross tumor volume MRI (GTVMR, red), clinical target volume MRI (CTVMR, green), planning target volume MRI (PTVMR, orange), and hybrid GTVMR+FET (blue), CTVMR+FET (purple), PTVMR+FET (yellow). For comparison, GTVMR and GTVMR+FET are shown together with contrast-enhanced T1-weighted imaging, T2/FLAIR, and FET-PET. All images are co-registered to each case; respective planning CT where TVMR and TVMR+FET are displayed separately. Figure courtesy of Nathaniel Barry et al and presented at RANZCR ASM 2023.
An overview of all radiation oncology target volume delineations on axial imaging. Each row represents the benchmarking cases: FET-PET11 case 1 (a), FET-PET1 case 2 (b), and FET-PET1 case 3 (c). Shown here are standard gross tumor volume MRI (GTVMR, red), clinical target volume MRI (CTVMR, green), planning target volume MRI (PTVMR, orange), and hybrid GTVMR+FET (blue), CTVMR+FET (purple), PTVMR+FET (yellow). For comparison, GTVMR and GTVMR+FET are shown together with contrast-enhanced T1-weighted imaging, T2/FLAIR, and FET-PET. All images are co-registered to each case; respective planning CT where TVMR and TVMR+FET are displayed separately. Figure courtesy of Nathaniel Barry et al and presented at RANZCR ASM 2023.
Nuclear medicine physicians were asked to assess a series of sample patient imaging datasets, with those assessments forming crucial components of each patient's treatment and follow-up processes. Barry and his colleagues revealed where the inconsistencies occurred in such assessments. They showed that such inconsistencies were reduced through appropriate feedback to clinicians, greatly increasing the quality of treatments being offered to glioblastoma patients.
Quantifying the impact of inter-clinician variability in image interpretation helps to inform the modeling to be performed on imaging data acquired during the trial, and it will help to optimize the use of FET-PET in future patient treatments, they explained.
Brain malignancies
Glioblastoma is the most common adult primary brain malignancy with a poor prognosis. Recent guidelines from the European Society for Therapeutic Radiology and Oncology (ESTRO) and the European Association of Neuro-Oncology (EANO) define gross tumor volume (GTV) as resection cavity after surgery plus any residual enhancing tumor on contrast-enhanced T1-weighted MRI (T1c), with expansion to clinical target volume (CTV) using a margin of 15 mm, the Perth team noted.
"Furthermore, changes on T2-weighted and T2 fluid-attenuated inversion recovery (FLAIR) that are felt to reflect nonenhancing tumor should be incorporated into the CTV," they pointed out. "There has been ongoing research into alternative target volume definitions and boost volumes to improve local disease control, with some studies investigating PET."
The radiation oncology credentialing program for the FIG study has resulted in all sites successfully passing all mandatory requirements, in parallel with completion of the nuclear medicine credentialling aspects. Radiation oncologists' variability was similar between MR and MRI+FET derived volumes.
Key findings
In the group's study presented in Brisbane, at least one radiation oncologist per site completed three benchmarking cases. First, radiation oncologists were instructed to delineate standard of care MRI-derived target volumes (TVMR). CTVMR expansion was 15 mm and PTVMR expansion was 3 mm.
Radiation oncologists were then instructed to incorporate FET-PET biological tumor volumes (BTVs) into revised adjuvant radiation target volumes (TVMR+FET). CTVMR+FET expansion was 10-15 mm and PTVMR+FET expansion was 3 mm.
Organs at risk were also delineated, Barry and colleagues noted. The supplied BTV for each case was delineated by a single expert nuclear medicine physician using a semiautomatic procedure; hence, the supplied BTV for incorporation into revised hybrid volumes was the same for all radiation oncologists.
The researchers obtained data from 19 radiation oncologists across 10 trial sites. Case reviews were conducted on 54 initial submissions, with eight additional requested resubmissions (n = 6, missing contour[s]; n = 2, contour data not registered to planning CT) and four conditional passes (n = 3, image registration misalignment; n = 1, missing contour[s]). Initial pass rate was 42/54 (78%).
Volume differences amongst the 19 radiation oncologists compared between TVMR and TVMR+FET were significant (p < 0.001) across all three credentialing cases, they reported. Brainstem (Dice similarity coefficient, DSC = 0.85) and eyes (left, right DSC = 0.90, 0.91) exhibited the highest spatial agreement for the organs at risk, whereas the optic chiasm (DSC = 0.41) exhibited the least, sometimes not overlapping between the radiation oncologists at all.
Editor's note: You can read the team's complete findings and dataset by going to the poster on the EPOS section for the RANZCR ASM 2023 on the ESR's website. Also, see the group's paper published on 11 August 2023 by the European Journal of Nuclear Medicine and Molecular Imaging.