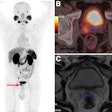
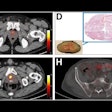
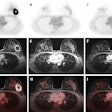
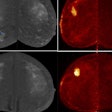
Blue Earth Therapeutics has secured an "innovation passport" from the U.K.'s Innovative Licensing and Access Pathway (ILAP) for its investigational therapeutic radiopharmaceutical lutetium-177 (Lu-177) rhPSMA-10.1 for treatment of metastatic prostate cancer.
The passport is the entry point to ILAP's process of accelerating time to market for investigational devices. ILAP coordinates a company's interaction with the U.K. Medicines and Healthcare products Regulatory Agency (MHRA) and the process of submitting marketing authorization applications, Blue Earth said.