An experimental new radiotracer is effective when used with PET/CT for detecting biochemically recurrent prostate cancer and may change patient management, according to research published online on 12 March in the Journal of Nuclear Medicine.
German researchers tested an experimental radiohybrid prostate-specific membrane antigen (rhPSMA) ligand, finding that it identified cancers in almost 73% of patients on PET/CT scans, with a detection rate of almost 62% in patients with prostate-specific antigen (PSA) levels of 0.2-< 0.5 ng/mL. The radiotracer also revealed local recurrence in 48.8% of patients.
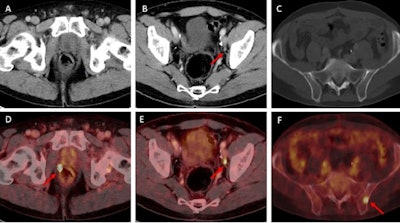

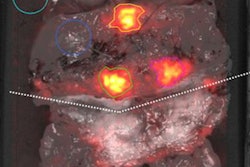
Examples of individual minor and major therapeutic change in patients with biochemical recurrence after radical prostatectomy undergoing F-18 rhPSMA 7.3 PET/CT examination.
(A, D) 70-year-old patient (PSA = 0.49 ng/mL) with F-18 rhPSMA 7.3 ligand uptake in the right prostatic bed (B, red arrow) without clear morphological correlate on corresponding CT. Therapeutic management was changed from radiation therapy of the prostatic bed to radiation therapy of the prostatic bed with simultaneous integrated boost being considered a minor change.
(B, E) 57-year-old patient presenting with biochemical recurrence (PSA = 1.0 ng/mL) seven years after radical prostatectomy. Fused F-18 rhPSMA 7.3 PET/CT shows focal PSMA ligand uptake in an unsuspicious lymph node adjacent to the left external iliac artery suspicious for singular lymph node metastasis. Therapeutic management was changed from radiation therapy of the prostatic bed and additional short-term androgen deprivation therapy to salvage lymphadenectomy.
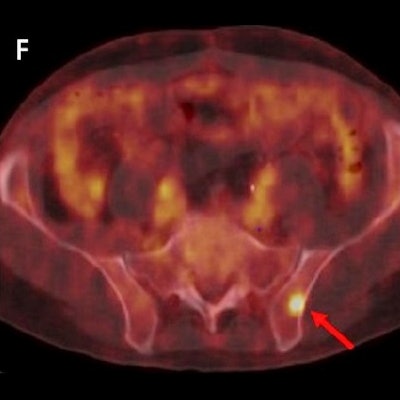
(C, F) 62-year-old patient presenting with biochemical recurrence (PSA = 0.3 ng/mL) a year and a half after radical prostatectomy. Fused F-18 rhPSMA 7.3 PET/CT shows focal PSMA ligand uptake in the left iliac bone without unequivocal morphological correlate. Therapeutic management was considered a major change (change from androgen deprivation therapy to stereotactic body radiation therapy of the singular bone metastasis). Images courtesy of the JNM.
"In this large population of patients with recurrent prostate cancer following radical prostatectomy and prior to any potential salvage therapy, [F-18] rhPSMA‐7.3 PET/CT offers high detection rates at least equal to those reported for [gallium-68] PSMA‐11," said researchers led by Dr. Isabel Rauscher of the Technical University of Munich, Germany.
Prostate cancer relapse following curative-intent primary therapy remains a considerable clinical challenge, with up to one‐third of patients experiencing biochemical recurrent disease. Part of the challenge is that conventional imaging as well as PET imaging for the localization of recurrence is limited in patients with low PSA levels.
However, a new class of rhPSMA ligands that use the superior nuclear properties of F-18 have been developed and are being tested in trials. The advantage of these agents is that they can be radiolabeled with either F-18 or with radiometal tracers like gallium-68 or lutetium-177 -- making possible both imaging and theranostic applications, according to the authors.
One such agent is F-18 rhPSMA-7.3, which is being developed by Blue Earth Diagnostics and is being studied in two multicenter phase III trials. The current study reports the first retrospective data on the radiopharmaceutical's efficacy in detecting cancer and its potential impact on clinical management in a cohort of patients with biochemical recurrence after radical prostatectomy prior to any salvage therapy.
Researchers analyzed 242 patients with a median PSA range of 0.2-60.8 ng/mL who underwent F-18 rhPSMA-7.3 PET/CT scans (Biograph mCT Flow, Siemens Healthineers). Further, patient management before and after PET was assessed by an interdisciplinary simulated tumor board.
Lesion detection rates were stratified by PSA, with the radiotracer showing higher levels of sensitivity in patients with higher PSA levels.
| Lesion detection rates by PSA level for F-18 rhPSMA-7.3 radiotracer | |
| PSA score | Lesion detection rate |
| 0.2-< 0.5 ng/mL | 61.8% |
| 0.5-< 1 ng/mL | 67.9% |
| 1 < 2 ng/mL | 81.1% |
| ≥ 2 ng/mL | 95.7% |
The researchers next assessed the impact of the scans on patient management using a simulated tumor board model. They found that use of F-18 rhPSMA-7.3 PET/CT changed therapeutic management in 153/242 patients (63.2%), with 54/242 (22.3%) considered major and 99/242 (40.9%) minor. Use of the radiotracer did not prompt any therapeutic changes in 64/242 patients (26.4%).
The authors noted several limitations to their study, including that clinical management before and after F-18 rhPSMA-7.3 PET/CT was assessed hypothetically, as part of a simulated tumor board, so that no information on actual implemented management change was available. Also, due to the retrospective character of the study, the analysis included patients from different external and internal referrers, where treatment approaches might be different.
Additional prospective evaluations are needed to prove the overall benefit of these management changes in patients, the researchers concluded.