
For patients who are exposed to ionizing radiation during diagnostic or therapeutic nuclear medicine examinations, the benefits of such procedures almost always far outweigh the radiation risks, but it's important to understand the risk of cancer induction for such patients. A research team from Sweden and the U.S. has now estimated the lifetime attributable risk (LAR) of cancer for patients of various ages and genders undergoing a range of nuclear medicine procedures.
Currently, the quantity typically used to estimate cancer induction and genetic effects from radiation is the effective dose. Defined by the International Commission on Radiological Protection (ICRP), the effective dose represents the total health detriment to radiation workers (18 to 65 years old) or the general public (all ages) as a result of radiation exposure.
Effective dose, however, does not provide risk estimates for patients undergoing medical procedures, where age and gender distributions can differ greatly from those of the general population. "The effective dose is accepted for comparisons of different diagnostic examinations and interventional procedures," said first author Martin Andersson, from Lund University in Malmö. "The problem is it has sometimes been used as a surrogate for risk to very different patient groups and even to individual patients."
 The study authors: (left to right) Keith Eckerman, Martin Andersson, and Sören Mattsson.
The study authors: (left to right) Keith Eckerman, Martin Andersson, and Sören Mattsson.Instead, Andersson and co-workers propose the use of LAR -- the additional cumulated probability of having a specific cancer over the patient's lifetime for a given radiation dose -- based on risk models from the U.S. Environmental Protection Agency (EPA). "The advantages of LAR values are they are gender and age specific, allowing risk estimations for specific patients or subgroups, thus better representing individuals in healthcare than effective dose," Andersson explained.
In this study, the researchers applied LAR methodology to estimate the risk of cancer occurrence (morbidity) or death (mortality) for patient subgroups undergoing nuclear medicine procedures. They compared their results with detriment-adjusted nominal cancer risk estimates, determined by multiplying the effective dose by the ICRP risk coefficients (4.1% per Sv for workers; 5.7% per Sv for the general population) (Physics in Medicine and Biology, 21 November 2017, Vol. 62:24, pp. 9177-9188).
Diagnostic procedures
The team first calculated age- and gender-dependent cancer morbidity and mortality risks for two diagnostic radiopharmaceuticals: F-18 FDG, the most prevalent PET tracer, which is used to assess glucose uptake in tumours; and Tc-99m-labeled phosphonates, which are employed in bone scintigraphy.
The calculated cancer morbidity risk, for example, from an F-18 FDG examination (using the recommended intravenous administration and a scan time per bed position of 2.5 minutes) was 0.0021 for a 5-year-old boy. This risk decreased to 0.0010, 0.0008, and 0.0003 for male patients 25, 50, and 75 years old, respectively.
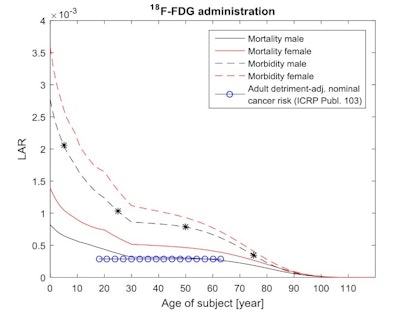 Age and gender dependent cancer morbidity and mortality risks from intravenous administered F-18 FDG. The adjusted nominal cancer risk to the Reference Person (18 to 65) for an adult working population is also presented. The stars indicate the radiation induced morbidity cancer risk for a male of 5 years (0.0021), 25 years (0.0010), 50 years (0.0008), and 75 years (0.0003).
Age and gender dependent cancer morbidity and mortality risks from intravenous administered F-18 FDG. The adjusted nominal cancer risk to the Reference Person (18 to 65) for an adult working population is also presented. The stars indicate the radiation induced morbidity cancer risk for a male of 5 years (0.0021), 25 years (0.0010), 50 years (0.0008), and 75 years (0.0003).For intravenously administered Tc-99m, the cancer morbidity risk was 0.00059 for a 5-year-old boy, 0.00034 at 25, 0.00027 at 50, and 0.00013 at 75. For both diagnostic radiopharmaceuticals, and for all patient ages, cancer morbidity and mortality risks were 10% to 30% higher for females than for males.
The predicted LAR cancer mortality risks for men ages 30 to 65 were numerically close to the nominal cancer risk for a working-age population (0.0003 for F-18 FDG and 0.00008 for Tc-99m). However, the results clearly illustrated the higher radiosensitivity of females and younger people.
Therapy assessments
Andersson and colleagues next examined two therapeutic radiopharmaceuticals. The first, I-131 iodide, is used for thyroid-related applications such as radioiodine ablation, a postsurgical treatment to eliminate thyroid cancer cells and thyroid tissue remaining after surgery.
The cancer morbidity risks from orally administered I-131 iodide, using recommended age-dependent administrations, were 0.082, 0.041, 0.029, and 0.012 for males ages 5, 25, 50, and 75. Here, morbidity and mortality risks were 20% to 30% higher for females than males at all ages.
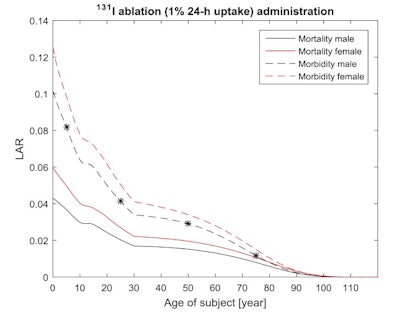 Age and gender dependent cancer morbidity and mortality risks from an orally administered I-131 iodide treatment. The stars indicate the radiation induced morbidity cancer risk for a male of 5 years (0.082), 25 years (0.041), 50 years (0.029), and 75 years (0.012).
Age and gender dependent cancer morbidity and mortality risks from an orally administered I-131 iodide treatment. The stars indicate the radiation induced morbidity cancer risk for a male of 5 years (0.082), 25 years (0.041), 50 years (0.029), and 75 years (0.012).The researchers also assessed Ra-223 dichloride, an alpha emitter used to treat castration-resistant prostate cancer. The cancer morbidity risks from six intravenous administered Ra-223 dichloride treatments, with 0.05 MBq/kg each treatment, were 0.31, 0.21, and 0.09 for 25-, 50-, and 75-year-old men.
The authors emphasize the LAR estimations are more suitable in healthcare situations involving individual patients or specific subgroups than the health detriment based on effective dose, which represents a population average.
"There is currently a great need for well-founded radiation risk estimates," Andersson said. "The new European Union Directive, which has to be transposed into member states' national legislation before 6 February 2018, emphasizes that adequate information relating to the benefits and risks associated with radiation dose from medical exposure should be given to patients."
Andersson noted the LAR predications in the paper used cancer statistics for U.S. populations. The authors have now initiated a new project, in cooperation with the EPA, using cancer risk estimations based instead on statistics from other populations. "The first step, which is currently ongoing in this project, is to implement the cancer risk calculations based on a Swedish population," he told medicalphysicsweb.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.




















