"A picture is worth a thousand words" -- a sentimental exclamation that may have slipped our lips many times. Whilst true for everyday snapshots depicting a friend's smile, children at play, animal stock, landscapes, or war zones, simple picture taking is no option for medicine.
Medical diagnosis, though being dependent in many ways on imaging information, cannot rest on a single picture. Even then, picture taking, or imaging, as the process of acquiring still pictures or series thereof, needs to be standardized. Furthermore, the imaging test needs to be selected for each patient a priori, based on the clinical indication, and, moreover, only certified imaging equipment can be employed. Last not least, images should be interpreted by successfully trained imaging experts.
You may comment, "We have got it all, why should we move away from what is good for us?" It is because imaging isn't good enough.
Despite the technical advances in noninvasive imaging ever since the discovery of x-rays by Wilhelm Conrad Röntgen in 1895, standalone imaging has remained limited in its applications. This holds true for imaging methods such as x-ray CT, ultrasound imaging, and nuclear medicine methods, e.g., SPECT. Even selected case scenarios for MRI or PET have not led to broadened clinical indications over the past decades. Of course, each structural, functional, or molecular imaging method comes with particular strengths and weaknesses (figure 1).
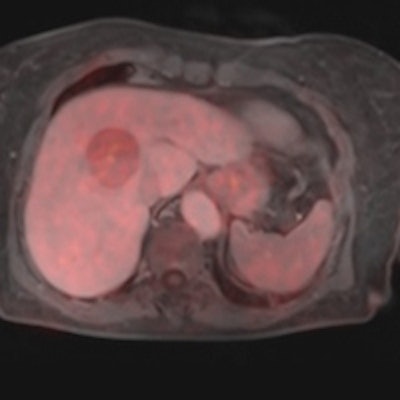
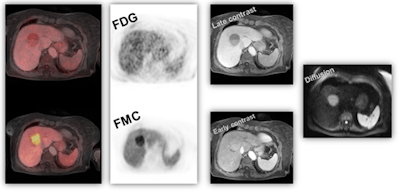 Figure 1: Dual tracer, multiparametric PET/MRI using F-18 FDG and F-18 FMC for noninvasive tumor characterization in a 70-year-old patient with suspected hepatocellular carcinoma (based on EORTC-criteria) prior to resection. Image courtesy of Dr. Markus Hartenbach, Medical University Vienna in Austria.
Figure 1: Dual tracer, multiparametric PET/MRI using F-18 FDG and F-18 FMC for noninvasive tumor characterization in a 70-year-old patient with suspected hepatocellular carcinoma (based on EORTC-criteria) prior to resection. Image courtesy of Dr. Markus Hartenbach, Medical University Vienna in Austria.It was with the physical combination of noninvasive imaging methods, such as SPECT/CT, PET/CT or, lately, PET/MRI that for the first time cross-specialty thinking sunk into the minds of the diagnostic clinical experts, and following clinical implementation, gave rise to documented evidence of increasing diagnostic accuracy.1,2 However, when presenting that imaging information in clinical tumor boards, or alike, it became clear that effective patient management mandates additional information that include pathology and data analysis methods.3 These fields, while being in active pursuit by several groups, could not be regarded independently any more, particularly when addressing severe illnesses, such as cancer or other metabolic diseases.
Conjoined assessment of morphology, metabolism, and pathology becomes even more important when assessing therapy response4 when lesions may respond biologically but no alteration of the morphology is detected (figure 2). To date, numerous novel and effective pharmaceuticals for therapeutic interventions are available. If chosen wisely, these therapies can be efficient.5 However, frequently an a priori patient selection for the most appropriate therapy is missing, mainly due to missing out on molecular imaging information. Therefore, antitumor therapies may lack efficiency.6
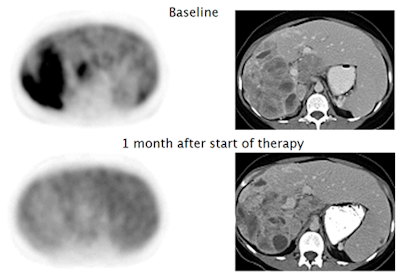 Figure 2: Patient with gastrointestinal stromal tumor (GIST), showing early metabolic response to Imatinib therapy (F-18 FDG PET, left side), without change in size of the tumor (CT, right side). Panels modified from (4).
Figure 2: Patient with gastrointestinal stromal tumor (GIST), showing early metabolic response to Imatinib therapy (F-18 FDG PET, left side), without change in size of the tumor (CT, right side). Panels modified from (4).Although continuous scientific improvements are being made and new therapeutic options become available, equally relevant improvements in outcome measures (e.g., overall survival, time to tumor progression, patients' well-being) are rarely observed. On the contrary, the economic burden for the healthcare systems increases steadily, both on an overall and an individual scale without matching benefit for the treated patients7 (figure 3).
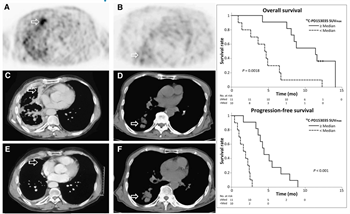
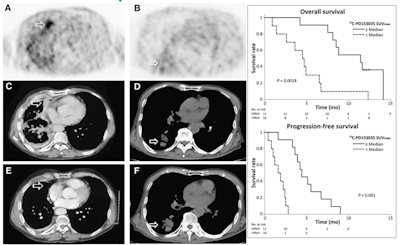
Figure 3: Baseline C-11 PD153035 PET before Erlotinib treatment in a patient with adenocarcinoma (A) and in a patient with squamous cell carcinoma (B). Corresponding CT images before treatment (C and D) and six weeks after treatment (E and F). Patient A presented with higher baseline SUVmax and marked radiographic improvement at six weeks after Erlotinib was initiated (A, C, and E). Tumor progression ultimately occurred at 5.5 months, and patient remained alive at follow-up after 11.8 months. However, patient B with lower baseline SUVmax had radiographic progression six weeks after Erlotinib was initiated (B, D, and F) and succumbed to his disease within 2.6 months. Modified from (10).
A major concern in the management of cancer patients is the gap between molecular understanding of cancer derived from modern cancer research and its application in diagnostic methods to stratify patients based on established biomarkers. If we want to expand on our understanding of disease mechanisms then shortcutting target identification and progress in the assessment of subsequent, novel therapeutic approaches are needed, as well as wider collaborative efforts across existing boundaries of medical disciplines and applied sciences (figure 4). These include, but shall not be limited to, biologists, pathologists, computer scientists, physicists, nuclear medicine specialists, and radiologists.
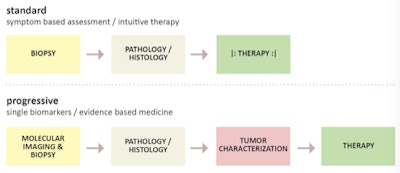 Figure 4: Standard and progressive approaches to patient-specific diagnosis and treatment. Figure courtesy of Ludwig Boltzmann Institute for Applied Diagnostics, LBI-AD, Vienna.
Figure 4: Standard and progressive approaches to patient-specific diagnosis and treatment. Figure courtesy of Ludwig Boltzmann Institute for Applied Diagnostics, LBI-AD, Vienna.Such cross-specialty engagement, including expertise in genomics, epigenomics, metabolomics, and proteomics can help advance novel target and biomarker definition, which, in turn, can help design new therapeutic approaches for diseases, such as cancer. In this setting, nuclear medicine molecular imaging techniques, such as SPECT and PET play a pivotal role8 by enabling repetitive noninvasive and quantitative imaging of the entire organism from small animals to humans, thus supporting the translation of our understanding of pathophysiological processes from mouse to men in a system biology approach.9
Applying cutting-edge, quantitative -omics technology to perform high throughput analyses to identify cancer genome or protein signatures in such a setting could provide potential molecular targets for nuclear imaging and thus help to validate data with unprecedented novelty.
An additional strength could be to facilitate the definition of novel biomarkers for both clinical practice of pathology and nuclear medicine diagnostic procedures as well as drug development for the treatment of cancer in clinical pharmacology, where after completion of these steps, novel suitable biomarkers could be defined and significant data could be present for being able to reach a decision upon proceeding with a translation into clinical trials.
The combination of -omics technologies, radiotracer development, and preclinical evaluation of imaging probes offers the unique possibility to define new targets and biomarkers while exploiting them directly in quantitative, molecular imaging. With this, the distribution of specific targets in vivo becomes possible and will lead to a deeper understanding of underlying molecular mechanisms in malignant diseases (figure 5).
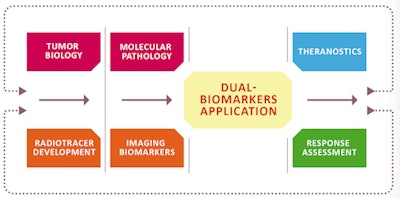 Figure 5: A conjoined assessment and understanding of tumor biology and molecular pathology enables the definition and validation of novel biomarkers for both diagnostic and therapeutic options, including therapy-response assessment. Figure courtesy of Ludwig Boltzmann Institute, lb:l, Vienna, Austria.
Figure 5: A conjoined assessment and understanding of tumor biology and molecular pathology enables the definition and validation of novel biomarkers for both diagnostic and therapeutic options, including therapy-response assessment. Figure courtesy of Ludwig Boltzmann Institute, lb:l, Vienna, Austria.Moreover, the sum of this information, including quantitative and imaging biomarkers, genetic predispositions and phenotypes, can help develop computer-assisted prediction models that will provide clinicians with a priori information on lesion classification (figure 6) and, possibly, predictive therapy response. It is with this sensible combination of recent technical and methodological advances that we need to look beyond simple imaging and move away from a "me > you" perspective to a collaborative and innovation-centric perspective on how to best assess disease and help patients most effectively.
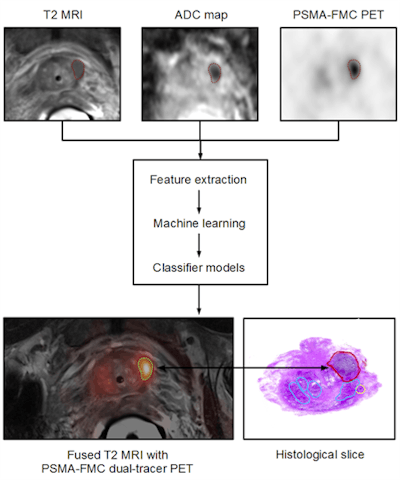 Figure 6: Patient with prostate cancer undergoing dual-tracer PET/MRI and subsequent ex vivo histopathology of the prostate. Combined PET/MRI includes T2-weighted MRI for anatomical referencing, Diffusion weighted imaging (DWI) for estimating cellular density and sequential Ga-68 PSMA and F-18 FCH imaging. Multiparametric image data processing supports the extraction of specific image features that are entered into a machine-learning (ML) process and subsequent computer-supported classifiers, which, based on sufficient and validated training efforts, permit the delineation and classification of tumor heterogeneity. Image courtesy of Laszlo Papp and Dr. Markus Hartenbach, Medical University Vienna, Austria.
Figure 6: Patient with prostate cancer undergoing dual-tracer PET/MRI and subsequent ex vivo histopathology of the prostate. Combined PET/MRI includes T2-weighted MRI for anatomical referencing, Diffusion weighted imaging (DWI) for estimating cellular density and sequential Ga-68 PSMA and F-18 FCH imaging. Multiparametric image data processing supports the extraction of specific image features that are entered into a machine-learning (ML) process and subsequent computer-supported classifiers, which, based on sufficient and validated training efforts, permit the delineation and classification of tumor heterogeneity. Image courtesy of Laszlo Papp and Dr. Markus Hartenbach, Medical University Vienna, Austria.If you would like to learn more about this approach to applied diagnostics, or become part of it, you may want to attend the next cross-specialty symposium, "Applied Diagnostics for Effective Cancer Treatment" (www.applied-diagnostics.eu) in Vienna in late September. There, you will find a forum of experts from both academia and industry in the field of molecular pathology, nuclear medicine, clinical pharmacology, and beyond who share this vision and like to engage in advancing effective and individualized treatments based on novel and validated diagnostic approaches.
Dr. Marcus Hacker is full professor of nuclear medicine at the department of biomedical imaging and image-guided therapy of the Medical University of Vienna. His research focuses on advanced molecular imaging concepts in cardiology and oncology and he is co-investigator of the Ludwig Boltzmann Institute for Applied Diagnostics.
Gerda Egger, PhD, is associate professor at the pathology department of the Medical University of Vienna and key researcher in the newly established Ludwig Boltzmann Institute for Applied Diagnostics. Her research has focused on the involvement of epigenetic aberrations in cancer and the establishment of epigenetic and molecular biomarkers for noninvasive tumor diagnostics.
Thomas Beyer, PhD, is full professor of physics of medical imaging at the Medical University of Vienna and co-developer of the PET/CT concept. His research is focused on quantitative and multimodality imaging in clinical research and routine.
The comments and observations expressed herein do not necessarily reflect the opinions of AuntMinnieEurope.com, nor should they be construed as an endorsement or admonishment of any particular vendor, analyst, industry consultant, or consulting group.
References
- Czernin J, Allen-Auerbach M, Nathanson D, Herrmann K. PET/CT in oncology: Current status and perspectives. Curr Radiol Rep. 2013;1:177-190.
- Czernin J, Ta L, Herrmann K. Does PET/MR Imaging Improve Cancer Assessments? Literature Evidence from More Than 900 Patients. J Nucl Med. 2014;55(suppl 2): 59S-62S.
- Aerts HJ, Velazquez ER, Leijenaar RT, et al. Decoding tumor phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5: 4006.
- Holdsworth CH, Badawi RD, Manola JB, et al. CT and PET: Early prognostic indicators of response to imatinib mesylate in patients with gastrointestinal stromal tumor. Am J Roentgenol. 2007;189(6):W324-330.
- Gaykema SB, Schroeder CP, Vitfell-Rasmussen J, et al. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin Canc Res. 2014;20(15):3945-3954.
- Gerlinger M, Rowan AJ, Horswell S, et al.. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883-892.
- Sullivan R, Peppercorn J, Sikora K, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011;12(10):933-980.
- Rosell R, Karachailou N. Lung cancer in 2014: Optimizing lung cancer treatment approaches. Nat Rev Clin Oncol. 2015;12(2):75-76.
- Weissleder R. Scaling down imaging: Molecular mapping of cancer in mice. Nat Rev Cancer. 2002;2(1):11-18.
- Meng X, Loo BW Jr, Ma L, et al. Molecular imaging with 11C-PD153035 PET/CT predicts survival in non-small cell lung cancer treated with EGFR-TKI: A pilot study. J Nucl Med. 2011;52(10):1573-1579.