Significant dosimetric errors in PET-guided planning of intensity-modulated radiation therapy (IMRT) can be avoided by using advanced segmentation algorithms, according to an article published in a September issue of Physics in Medicine and Biology. Use of these algorithms helps maintain patient safety and quality in a radiation oncology department.
Researchers from the French National Institute of Health and Medical Research (INSERM) in Brest determined that the advanced, automated methods outperformed simpler, threshold-based approaches when they were used to delineate tumor volumes in a series of simulated PET images (Phys Med Biol, 7 September 2012, Vol. 57:17, pp. 5381-5397).
"In terms of dosimetric accuracy, simple threshold-based methods may be sufficient for the delineation of PET gross tumor volume in homogeneous PET uptake cases. However, our study also demonstrates that significant dosimetry errors can be avoided by using more advanced image segmentation methods, especially when considering heterogeneous uptake volumes," said senior author M. Dimitris Visvikis, PhD, the director of research of the Laboratoire de Traitement de l'Information Médicale of INSERM U650.
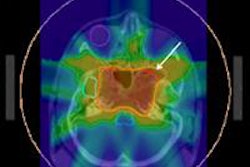
 The differences in segmentation algorithm performance for a simulated lung tumor with heterogeneous PET tracer uptake. Two sets of data were generated by adaptive thresholding as two users were instructed to each select a background region of interest, generating two different volumes.
The differences in segmentation algorithm performance for a simulated lung tumor with heterogeneous PET tracer uptake. Two sets of data were generated by adaptive thresholding as two users were instructed to each select a background region of interest, generating two different volumes.While IMRT can produce treatment plans that conform radiation isodoses closely to the shape of a target volume and steer dose away from surrounding healthy tissue, this capability places an increased onus on the accurate delineation of the tumor volume. In the case of PET-guided delineation, the superiority of advanced, automatic segmentation algorithms over manual and semiautomatic threshold-based delineation has been already documented in peer-reviewed published literature.
"The advantage of such automatic approaches is an improved robustness and reproducibility, as well as accurate handling of heterogeneous tumor radiotracer distributions," Visvikis said.
The algorithms also save time over semiautomated and manual approaches. But in spite of these advantages, the dosimetric impact of the different segmentation approaches on IMRT plans remained unknown, motivating the researchers' study.
Algorithm comparison
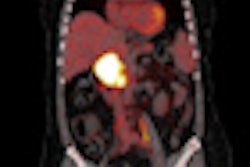
The researchers compared the dosimetric consequences of four segmentation algorithms using simulated FDG-PET images of three head and neck cancer cases and three lung cancer cases. For each case, varying configurations of contrast and heterogeneity were considered. The simulated images provided a ground truth planning target volume for performance assessment and control over the simulated tumor size, location, and FDG uptake, enabling algorithm performance evaluation over a range of tumor characteristics.
The six image sets were generated by Monte Carlo simulations on two digital phantoms, one for each anatomical location. Accompanying CT images of the same anatomy also were simulated to enable dose calculation.
Two simple algorithms using fixed and adaptive thresholds were assessed against two advanced, automated techniques. The first was a previously reported fuzzy C-mean clustering algorithm. The second was a fuzzy locally adaptive Bayesian (FLAB) algorithm, developed by the researchers and already used for clinical research in their radiotherapy department and several collaboration sites worldwide. The algorithms were used to delineate the gross tumor volume and a standard 3-mm expansion was used to convert gross tumor volumes directly into planning target volumes, neglecting setup errors and patient motion.
Fuzzy algorithms give superior plans
No statistically significant difference in algorithm performance was detected for segmentation of homogeneous FDG uptake, but the automated algorithms did produce superior coverage of the ground truth planning target volume in lesions with heterogeneous uptake. The FLAB algorithm resulted in 89.1 ± 6.4% of the planning target volume receiving at least 95% of the prescribed dose and 95% of the planning target volume receiving at least 89.4 ± 6.1% of the prescribed dose, when averaged over all heterogeneous lesions. The fuzzy C-mean clustering algorithm produced similar results.
In comparison, the highest values produced by the threshold-based algorithms for the same dosimetric parameters were 75.4 ± 13.8% and 71.0 ± 21.5%, respectively. This inferior dose coverage resulted from a consistent underestimation of the volume by the simpler algorithms.
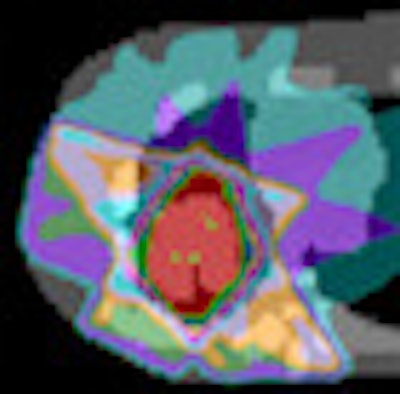
 Dosimetric impact of different segmentation algorithms for a simulated lung tumor with heterogeneous PET tracer uptake.
Dosimetric impact of different segmentation algorithms for a simulated lung tumor with heterogeneous PET tracer uptake.Fixed threshold segmentation was also found to perform poorly when used to delineate low-contrast lesions of FDG uptake, sometimes grossly overestimating tumor volume. For example, the volume of one head and neck case with a lesion uptake-to-background ratio of 1.8:1 was overestimated by 333%. In such cases, the resulting volumes were deemed too inaccurate to justify calculation of a treatment plan.
According to Visvikis, incorporation of the automated algorithms into commercial treatment planning systems will best facilitate their introduction to clinical practice, enabling the associated dosimetric benefits to be widely realized.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.