To ignore the issue of incidental findings is unacceptable. Imaging research centers must manage these findings as if they were a medical issue, and they need to establish specific and detailed guidelines and protocols to manage abnormal findings, ranging from life-threatening to minor.
These are among the key recommendations of a U.K.-wide initiative undertaken by the Royal College of Radiologists (RCR) and the Scottish Imaging Network: A Platform for Scientific Excellence (SINAPSE). Their report, "Management of Incidental Findings Detected During Research Imaging," was published last week and includes the opinions of researchers, ethicists, patient groups, physicians, and professional, regulatory, and funding bodies from the U.K. and also other European countries.
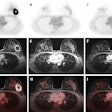
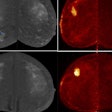
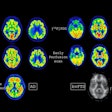
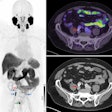
Medical imaging -- specifically CT, MRI, ultrasound, and molecular imaging -- is used increasingly in research involving participants who are presumed to be healthy. These modalities are identifying subtle abnormalities that might have otherwise gone unnoticed. Approximately 3% to 12% of brain imaging examinations performed identify abnormalities. On body imaging, especially cardiac CT and colonography, the rate can be as high as 30%.
|
Practical advice for researchers
Source: "Management of Incidental Findings Detected During Research Imaging" |
This poses a dilemma, because as much as 43% of research imaging in the U.K. is undertaken by scientists without medical training, often in research centers that are remote from radiology departments or hospitals, according to the report. A review of all European agencies guidance indicates that management of incidental pathology is inconsistent, limited, and nonspecific.
The study also determined that the few existing standards that do exist are not being applied consistently. Practices for recognizing, interpreting, and managing any medical or other consequences of incidental findings vary widely -- from doing nothing and not informing the individual, to having them interpreted by a radiologist and referring the individual to his or her physician or a specialist.
While having a qualified radiologist review all research images would be the best method to identify incidental findings, research imaging does not have this obligation, the authors of the report added. To initiate this practice would create serious practical implications in view of the shortage of radiologists in the U.K., and the fact that many research imaging centers are not linked with healthcare facilities where radiologists work.
A lack of evidence on which to base management of incidental findings exists. Lack of evidence was identified in these topics and situations:
- Evaluation of harm versus benefit of notifying participants with abnormal findings, especially if they prove to be incorrect
- The rate of false positives
- How often failure to notify a participant might actually cause a serious health problem to him/her
- The percentage of participants who do not want to be notified
- The percentage of individuals who would decline participation if they were told that they would not be notified if the imaging exams they agreed to have showed abnormal findings
- Methods to best refer patients to physicians, and methods of obtaining radiology review, including transfer of exams from remote locations
- The administrative time this would add to a researcher's job and the costs that would be incurred
The U.K. Department of Health and the National Research Ethics Service offer little guidance. The report quotes these agencies as stating that research participants "should be made aware of possible disadvantages and risks of taking part in research," and that "risks should be outlined, including discovery of another condition of which they were unaware that might have medical or insurance implications."
A list of the authors' main recommendations is given in the table. The 60-page report may be read in its entirety on the Royal College of Radiologists' website.