The introduction of MR-guided radiotherapy into clinical practice enables direct visualization of structures of interest in the treatment position, with high soft-tissue contrast. This opens up the possibility of adaptive replanning to compensate for changes in the patient's anatomy or position. It also creates an urgent need for dosimetry protocols and tools that are unaffected by the presence of a magnetic field.
With this aim, researchers in Greece have developed a method for overall dosimetric evaluation of a commercially available MR linear accelerator (MR-linac) system. Their approach combines 2D film measurements with 3D dose readout using passive polymer gel dosimetry. They have now demonstrated treatment plan verification of a cranial intensity-modulated radiotherapy (IMRT) case following virtual couch shifting.
Virtual couch shifts (or equivalent isocenter shifts) are used in MR-linac systems to account for patient setup errors. The required shift is calculated by registering a pretreatment MR scan with the planning CT, and a new plan is created using either an adapt-to-position or an adapt-to-shape method. This adapted plan should provide a dose distribution as close as possible to the original plan. But this is not always the case. Adapted plans may fail to meet the clinical dose constraints, necessitating extensive quality assurance (QA) of any new plan.
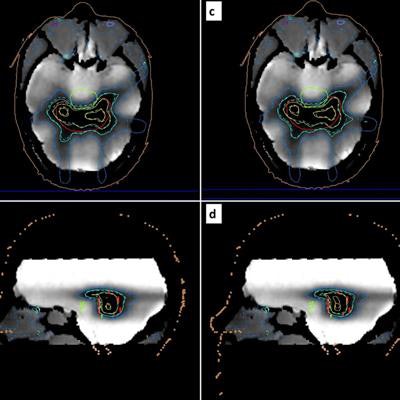
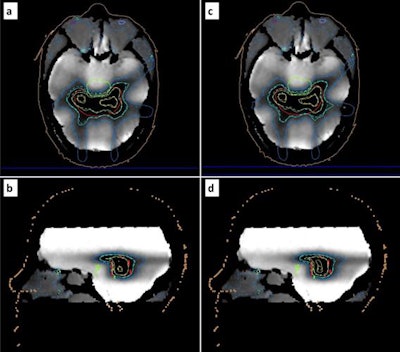 Reconstructed maps from a postirradiation MR scan of the gel-filled phantom. Corresponding relative dose measurements are superimposed (dashed isolines) for comparison with relative dose calculations (solid isolines) related to the adapted (a, b) and original (c, d) treatment plans. Blue, turquoise, green, and yellow isolines correspond to the 40%, 60%, 80%, and 100% isodose, respectively. The PTV, organ at risk, and external surface are depicted by red, green, and brown contours, respectively. Image courtesy of Pappas and colleagues.
Reconstructed maps from a postirradiation MR scan of the gel-filled phantom. Corresponding relative dose measurements are superimposed (dashed isolines) for comparison with relative dose calculations (solid isolines) related to the adapted (a, b) and original (c, d) treatment plans. Blue, turquoise, green, and yellow isolines correspond to the 40%, 60%, 80%, and 100% isodose, respectively. The PTV, organ at risk, and external surface are depicted by red, green, and brown contours, respectively. Image courtesy of Pappas and colleagues.Polymer gels provide an ideal platform for MR-linac QA, since they are not affected by magnetic fields. In addition, the MR component of the treatment system can be used to read out the irradiated gels.
"Gel dosimeters 'trap' dosimetric information in 3D within their irradiated volume; this information can then be extracted by MR scanning the irradiated gels," explained first author Evangelos Pappas, PhD, from the University of West Attica. "The 3D spatial resolution can reach values of 1 mm3. In addition, there are no wires or any ferromagnetic components that could introduce uncertainties."
Personalized process
The proposed QA protocol is based on the use of PseudoPatient head phantoms, in which a bone-equivalent phantom is 3D printed based on the patient's planning CT. In this study, the researchers used two identical phantoms: one incorporating an insert for 2D film dosimetry; the other filled with polymer gel that acts both as a 3D dosimeter and a soft-tissue equivalent. (Physics in Medicine & Biology, November 2019, Vol. 64:22, 225009).
"The PseudoPatient is probably the only phantom that provides both CT and MRI contrast," Pappas said. "Therefore, a PseudoPatient filled with polymer gel can be used to test the whole clinical MR-linac workflow, including adaptation. Moreover, since this phantom is constructed using human CT scans, one can incorporate realistic organs at risk, allowing the whole process to mimic a clinical case."
To test their approach, Pappas and collaborators created a seven-beam IMRT plan for a hypothetical C-shaped brain lesion partly surrounding the brainstem (the organ at risk). They placed each phantom on the Elekta Unity MR-linac's treatment couch and acquired MR images. The treatment planning system calculated the required virtual couch shifts and created an adapted treatment plan, using the adapt-to-position strategy. Finally, they delivered the adapted plan to each respective phantom.
For the PseudoPatient with the film insert, measurements agreed well with dose calculations for both the adapted and the original plans.
Prior to the gel measurements, the researchers evaluated the dose response characteristics of the gel under irradiation and readout conditions and saw a linear response in the investigated dose range. Immediately after plan delivery, they MR scanned the phantom to read out the dosimetry gel. Measurements corresponded well with the adapted and original calculated dose distributions. Dose volume histograms were in excellent agreement with calculations for the planning target volume (PTV), though minor discrepancies were seen for organs at risk.
The team also calculated several relative dose volume metrics for the PTV. These confirmed the good agreement (within ± 4%) between adapted and delivered plans. For this specific case and adaptation method, no considerable discrepancies were detected between the adapted and original plans.
Real-time video
As a proof of concept, the researchers also monitored dose accumulation in real-time by continuously MR scanning the gel-filled phantom during radiation delivery.
"This is the world's first video demonstrating experimentally in quasi-real-time the dose deposition process within the PTV, during delivery of a clinical IMRT plan," Pappas told Physics World. "Elekta Unity users can not only 'see' the human anatomy during treatment, but they can also 'see' the dose deposition as it accumulates during irradiation within a passive/integrating dosimeter."
The researchers concluded that polymer gel is an excellent candidate for end-to-end MR-linac QA, and that the study demonstrates that the Unity can deliver treatments with superb spatial and dosimetric accuracy. They noted that all tests were completed within approximately 30 minutes (plus 30 minutes of MR scanning for the 3D dosimetry readout), suggesting that this method could be used for routine QA. Next, the team plans to develop solutions for adapt-to-shape plan adaptation and for moving targets.
Tami Freeman is the medical and biophysics editor for Physics World.
© IOP Publishing Limited. Republished with permission from Physics World, a website that helps scientists working in academic and industrial research stay up to date with the latest breakthroughs in physics and interdisciplinary science.