SPL Medical of the Netherlands has received approval in Germany to test its Ferrotran (ferumoxtran-10) MRI contrast agent in a clinical trial.
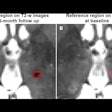
Researchers from Germany, Switzerland, and the Netherlands will lead a study evaluating the viability of Ferrotran, formerly Combidex, for detecting lymph node metastases in 180 patients with prostate cancer. The contrast agent is capable of detecting metastases as small as 2 mm in the lymph nodes, which is considerably smaller than the 7-mm threshold detectable by standard MRI and CT scanners, according to SPL.
The outcome of this trial will give new insights into the value of Ferrotran in the early detection of lymph node metastasis from prostate cancer, principal investigator Dr. Bernd Hamm, chair of radiology at the Charité, Humboldt-Universität zu Berlin, said in a statement.
The study was approved by the German Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte). SPL Medical is a spin-off of Radboud University Medical Center in the Netherlands.