An artificial intelligence (AI) algorithm can automatically quantify coronary and other arterial calcification from routine chest CT studies, enabling cardiovascular risk assessment to be performed on these patients, Dutch researchers reported at the RSNA 2017 meeting.
After testing their internally developed deep learning-based algorithm, the researchers found fully automatic scoring of arterial calcifications correlated highly with manual scoring results -- even in most ungated routine chest CT studies with severe pathologies, said Nikolas Lessmann from the University Medical Center Utrecht in the Netherlands.
"Automatic reporting of the arterial calcification burden in chest CT is feasible, regardless of the original indication for the scan," Lessmann said. He presented the findings during a scientific session at the conference in Chicago.
Common cause of death
Cardiovascular diseases are the most common cause of death worldwide, and accounted for more than 30% of all deaths in 2015. Coronary artery calcium (CAC) is a robust predictor of cardiovascular events in asymptomatic individuals, and CAC scoring can be performed on dedicated cardiac CT as well as ungated chest CT exams, Lessmann said.
The 2016 guidelines from the Society of Cardiac Computed Tomography (SCCT) and the Society of Thoracic Radiology (STR) noted the incorporation of CAC into all noncontrast chest examination reports represents a potential major advance in the early detection and treatment of coronary artery disease. Unfortunately, reporting arterial calcification on chest CT does come with some drawbacks, Lessmann said.
Arterial calcification is usually an unrequested finding, and therefore creates additional workload. What's more, it can be time-consuming to manually perform the quantification process, he said.
"Nonquantitative alternative scoring methods have been discussed, but automatic detection and quantification is another potential alternative," Lessmann said.
The 2016 SCCT/STR recommendations call for CAC to be evaluated and reported on all noncontrast chest CT examinations. In addition, the societies also said it may be reasonable to evaluate and report thoracic aortic calcification (TAC) on these studies, Lessmann said.
A deep-learning algorithm
The University Medical Center Utrecht researchers wanted to follow up on their previous work to develop an automatic, deep learning-based algorithm for quantifying coronary and other arterial calcifications on chest CT. The convolutional neural network, which was trained using 1,000 manually annotated low-dose chest CT studies from the U.S. National Lung Screening Trial (NLST), was originally intended to assess cardiovascular risk on lung cancer screening exams. Lessmann also shared the group's experience with the algorithm for automatically calculating CAC on low-dose CT in another presentation at RSNA 2017.
In this study, the team wanted to see if it could also handle clinical chest CT studies, Lessmann said.
"In contrast to screening scans, many of these patients have acute pulmonary symptoms or other abnormalities that the automatic system needs to cope with," he told AuntMinnieEurope.com.
They retrospectively tested the software on 290 consecutive routine chest CT scans acquired in 2015 at their hospital. The patients had an average age of 61.1 ± 13.9 years and 58% were male. CT scans were conducted on one of three different Philips Healthcare CT scanners, including an iCT 256-row (roughly 30% of the cases), Brilliance 64-row (61% of the cases), or Mx8000 IDT 16/Brilliance 16P 16-row systems (9% of the cases).
All exams were performed without gating and intravenous contrast. The helical scans were reconstructed using filtered back projection (FBP) reconstruction, and were acquired, if possible, with inspiratory breath-hold. Other scanning parameters included a tube voltage of 100 kVp or 120 kVp, a slice thickness of 0.9 mm or 1.0 mm, and a 0.7 mm increment, according to Lessmann.
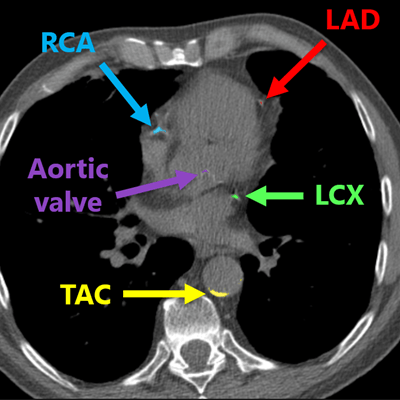
In all scans, manual annotations were performed for calcifications that were found in the left anterior descending artery (LAD) inclusive of the left main coronary artery, as well as the left circumflex artery (LCX), the right coronary artery (RCA), the visible part of the aorta, the aortic valve, and the cardiac valve. However, 16 (6%) of the scans had insufficient image quality and were excluded from the research project, Lessmann said. It took between five to 60 minutes to manually annotate each study.
CAC was found in 61% of the patients, while thoracic aortic calcium (TAC) was present in 78% of patients. Calcification was found in the aortic valve in 32% of patients and in the mitral valve in 13% of patients.
High correlation
After applying the algorithm to the studies to detect and quantify calcifications, the researchers group then used intraclass coefficient (ICC) analysis to determine how well the software's results correlated with the manually generated scores.
| Correlation between deep-learning and manual calcification volume scores | |
| Correlation (intraclass coefficient) | |
| CAC | 0.93 (Confidence interval: 0.90-0.94) |
| TAC | 0.99 (CI: 0.99-0.99) |
| Mitral valve calcifications | 0.94 (CI: 0.92-0.95) |
| Aortic valve calcifications | Aortic valve calcifications 0.79 (CI: 0.92-0.95) |
The team also found the algorithm had an 82% agreement with manual scoring in terms of patient risk category derived from calculated calcification scores. In 15% of cases, there was one category difference between the two methods.
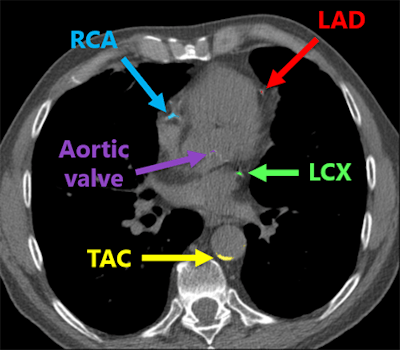 Deep-learning algorithm correctly detected and labeled calcifications on a routine chest CT scan. All images courtesy of Nikolas Lessmann.
Deep-learning algorithm correctly detected and labeled calcifications on a routine chest CT scan. All images courtesy of Nikolas Lessmann.This performance yielded a linearly weighted kappa of 0.82, indicating an "excellent" level of reliability, Lessmann said.
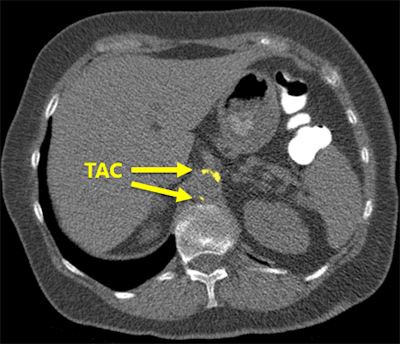 The software correctly detected an example of thoracic aortic calcification near the base of the lungs.
The software correctly detected an example of thoracic aortic calcification near the base of the lungs."We found that the system struggles with extreme cases, but overall performs very well," he said. "This can potentially enable fully automatic assessment of cardiovascular risk in all patients who receive a chest CT scan for whatever indication."



















