It's official: After months of intense lobbying and wrangling, the use of some linear gadolinium contrast agents in MRI will be restricted, and others are to be pulled from the market, following confirmation on Friday that the European Medicines Agency (EMA) has approved recommendations made on 7 July by the Pharmacovigilance Risk Assessment Committee.
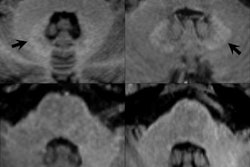
The decision was spelled out in an EMA press release about this week's meeting of the Committee for Medicinal Products for Human Use (CHMP). The agency said that it had completed the protracted review of gadolinium contrast agents, and it found that gadolinium deposition occurs in brain tissues following the use of certain contrast agents.
"There is currently no evidence that gadolinium deposition in the brain has caused any harm to patients; however, EMA has recommended restrictions for some intravenous linear agents in order to prevent any risks that could potentially be associated with gadolinium brain deposition," the EMA wrote in the statement.
Two intravenous linear agents, gadoxetic acid (Primovist in Europe; Eovist in the U.S., Bayer HealthCare Pharmaceuticals) and gadobenic acid (MultiHance, Bracco), will remain available as these agents undergo hepatic uptake and can be used for imaging poorly vascularized hepatic lesions, especially in delayed-phase imaging, that cannot be adequately studied with other agents.
| European Medicines Agency recommendations | ||
| Product | Type (formulation) | Recommendation |
| Artirem/Dotarem (gadoteric acid) | Macrocyclic (IV) | Maintain |
| Artirem/Dotarem (gadoteric acid) | Macrocyclic (intra-articular) | Maintain |
| Gadovist (gadobutrol) | Macrocyclic (IV) | Maintain |
| Magnevist (gadopentetic acid) | Linear (intra-articular) | Maintain |
| Magnevist (gadopentetic acid) | Linear (IV) | Suspend |
| MultiHance (gadobenic acid) | Linear (IV) | Restrict use to liver scans |
| Omniscan (gadodiamide) | Linear (IV) | Suspend |
| Optimark (gadoversetamide) | Linear (IV) | Suspend |
| Primovist (gadoxetic acid) | Linear (IV) | Maintain |
| ProHance (gadoteridol) | Macrocyclic (IV) | Maintain |
All other intravenous linear products will be suspended in the European Union, and they are listed below:
- Gadodiamide, sold under the trade name Omniscan by GE Healthcare
- Gadopentetic acid, marketed as Magnevist by Bayer
- Gadoversetamide, sold as Optimark by Guerbet
However, the intra-articular formulations of the linear agent gadopentetic acid will continue to be available because the dose of gadolinium that is required for these scans is very low, EMA explained.
Another class of gadolinium agents, macrocyclic agents are more stable and have a lower propensity to release gadolinium than linear agents, the EMA continued. These products can still be used in their current indications but in the lowest doses that enhance images sufficiently and only when unenhanced body scans are not suitable. These include the following:
- Gadobutrol (Gadovist, Bayer)
- Gadoteric acid (Artirem/Dotarem, Guerbet)
- Gadoteridol (ProHance, Bracco)
Lifting of suspensions/restrictions
The suspensions or restrictions on linear agents can be lifted if the companies can provide evidence of new benefits in an identified patient group that outweigh the risk of brain deposition or if the companies can modify their products so they do not release gadolinium significantly or cause its retention in tissues, the agency noted.
The EMA noted the following information should be given to patients:
As a precaution, doctors will stop using some contrast agents given into the vein, while some others will only be used when other agents are not suitable (e.g., for liver scans). If you need a scan with a gadolinium contrast agent to help in your treatment, your doctor will use the lowest dose required for a clear image. If you have any questions about your scan, speak to your doctor.
In its information for healthcare professionals, EMA stated that gadolinium deposition in the brain has been confirmed by mass spectrometry and increases in signal intensity in brain tissue.
"Data on stability, as well as in vitro and nonclinical studies, show that linear gadolinium agents release gadolinium from the ligand molecules to a greater extent than macrocyclic agents. No adverse neurological effects, such as cognitive or movement disorders, have been attributed to gadolinium deposition in the brain with any gadolinium agents," the agency noted.
Healthcare professionals should use gadolinium contrast agents only when essential diagnostic information cannot be obtained with unenhanced scans, and they should always use the lowest dose that provides sufficient enhancement for diagnosis, EMA added. The product information for gadolinium contrast agents remaining on the EU market will be updated accordingly, and healthcare professionals in the EU will be sent a letter with information about the EMA's review of gadolinium contrast agents.
The CHMP meeting took place between 17 and 20 July. The final stage of the procedure is the adoption by the European Commission of a legally binding decision applicable in all EU member states.