Cardiac PET is used to measure tissue viability, myocardial perfusion, and functional parameters such as ejection fraction (EF), the percentage of blood pumped out each time the heart contracts. The EF is an important measure of heart function and provides valuable information for diagnosis and treatment of patients with coronary artery disease. Respiratory motion during PET scans, however, can degrade image resolution and affect the accuracy of EF measurements.
Cardiac electrocardiogram gating is employed in the clinic to study the contractile function of heart, by dividing the beating cycle into a number of phases. Now, researchers from Oakland University, Duke University, and Johns Hopkins University have developed a method for respiratory motion compensation cardiac PET, including a respiratory gating technique and a 4D motion-corrected image reconstruction algorithm. They investigated the effect of this motion correction approach on measurements of end-diastolic volume (EDV) and end-systolic volume (ESV), and on the detectability of an abnormal EF (Physics in Medicine and Biology, 7 June 2017, Vol. 62:11, pp. 4496-4513).
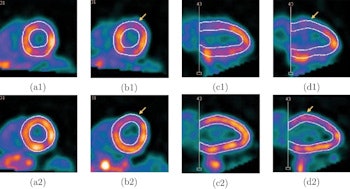
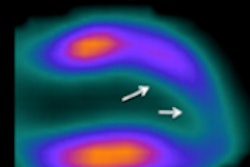
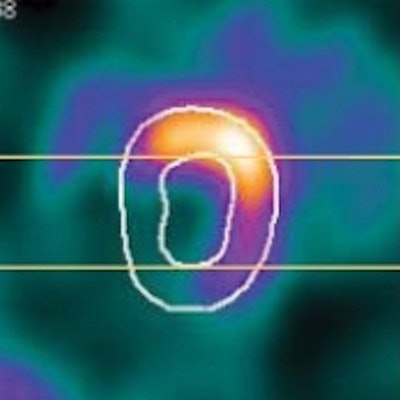
Screenshots of end systolic heart images with normal (a) and abnormal (b) LV motion in the short-axis view, and with normal (c) and abnormal (d) LV motion in the vertical long-axis view, without (1) and with (2) respiratory motion correction, from the standard XCAT phantom simulation. The arrows indicate the location of the motion defect.
"Respiratory motion degrades PET image quantification in the thoracic region, especially cardiac imaging," explained first author Jing Tang. "Respiratory gating reduces motion blurring but suffers from low statistics. In addition, the complexity of hardware-based tracking procedures limits the application of respiratory gating in clinics. This prompted us to develop new respiratory gating and motion compensation approaches."
Simulation study
To study the effect of their motion correction scheme, Tang and colleagues simulated cardiac PET data for normally and abnormally beating hearts, using the standard XCAT phantom and two individual-specific XCAT phantoms (with large and small respiratory motion). They measured the EDV and ESV from images reconstructed with and without motion correction, and used these values to calculate the respective EFs (EF is equal to (EDV-ESV)/EDV).
Comparing images reconstructed with and without respiratory correction revealed a blurring caused by respiratory motion, which led to thicker myocardium and smaller measured ventricle volume in uncorrected images. In the standard XCAT simulation, EDV and ESV were underestimated from images without motion correction, for both normal and abnormal hearts. Correction reduced the blur and increased all measured volumes.
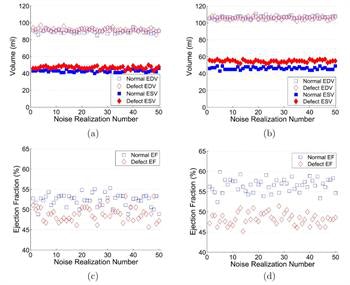
Estimated EDVs and ESVs from the images of normal and abnormal hearts reconstructed without (a) and with (b) respiratory motion correction and the corresponding EFs without (c) and with (d) respiratory motion correction, from the standard XCAT phantom simulation.
This motion-induced underestimation was also shown by an increase in the normalized root mean square error (NRMSE), which reflects bias with respect to the true value. For the normal heart, NRMSEs of EDV and ESV measured without motion correction were 21.9% and 12.8%, while errors with motion correction were reduced to 7.5% and 6.3%, respectively. In the abnormal heart, the NRMSEs of EDV and ESV were 21.7% and 20.7% without motion correction, and 9.6% and 7.6% with correction.
The difference between normal and abnormal ESV values increased after correction, from 4 ml to 8.4 ml. This led to an improved signal-to-noise ratio between normal and abnormal EFs, from 1.45 to 3.86 -- demonstrating that respiratory motion correction leads to easier detection of an abnormal EF.
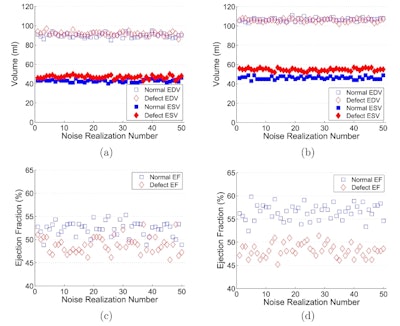
The effect of respiratory motion correction was also beneficial in the two customized XCAT phantoms, reducing the NRMSEs and improving the signal-to-noise ratio between normal and abnormal EFs, from 0.28 to 3.45 for one and from 0.59 to 2.21 for the other.
Comparing the customized phantoms showed the relative differences between EDV (in normal and abnormal cases) with and without motion correction were larger in the phantom with larger respiratory motion (about 2 cm) than in the phantom with 0.7 cm motion amplitude. The same trend was seen in ESV measurements. These findings demonstrate the larger the respiratory motion, the greater impact motion correction has on detection of abnormal EF. The authors note for motion amplitudes as small as 0.7 cm, correction still significantly improved detection of abnormal EF.
Patient study
Tang and colleagues also evaluated the effect of their respiratory motion correction technique on ECG-gated FDG cardiac PET data of two patients. Rather than using hardware-based respiration tracking, the proposed gating technique extracts the respiratory motion signal from the list-mode PET data itself. The researchers employed this data-driven motion signal and the electrocardiogram triggers in the list-mode data to perform dual respiratory and cardiac gating for each patient. Images reconstructed without respiratory motion correction were blurred, resulting in a smaller left ventricle chamber than seen in motion corrected images with reduced blur.
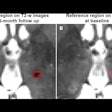
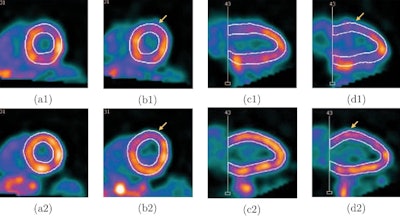
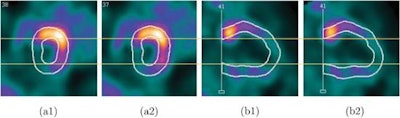 Screenshots of end systolic heart images in short-axis view (a) and vertical long-axis view (b), without (1) and with (2) respiratory motion correction, from the patient 1 study.
Screenshots of end systolic heart images in short-axis view (a) and vertical long-axis view (b), without (1) and with (2) respiratory motion correction, from the patient 1 study.As in the simulations, respiratory motion correction increased measured volumes and EF in the patient study. EDV, ESV, and EF for patient 1 were 54 mL, 31 mL, and 43%, respectively, without motion correction, and 65 mL, 33 mL, and 49% with motion correction. For patient 2, the EDVs, ESVs, and EFs without and with motion correction were 60 mL, 33 mL, and 45%, and 68 mL, 34 mL, and 50%, respectively.
The findings of this patient study reiterate the benefit of respiratory motion correction for EF measurement by cardiac PET. "We are now studying and correcting the effect of respiratory motion in cardiac imaging for other purposes, for example, dynamic imaging for myocardial blood flow measurement," Tang told medicalphysicsweb.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.