MR-guided high-intensity focused ultrasound (HIFU) is safe for breast tumor ablation, offering an even less invasive alternative to lumpectomy -- as well as other forms of ablation -- for women with early-stage invasive breast cancer, according to a new study published online on 6 February by European Radiology.
The study findings are good news, especially as breast cancer treatment continues to evolve, researchers at the University Medical Center Utrecht in the Netherlands wrote. Breast-conserving therapy (that is, lumpectomy) with additional radiotherapy is the current standard-of-care for early-stage breast cancer; a range of ablation techniques such as cryoablation, radiofrequency ablation, or microwave ablation offer lumpectomy alternatives. But even these techniques require that a probe be inserted into the breast tumor.
That's why HIFU with MR guidance is a promising option, according to study lead author Dr. Laura Merckel and colleagues.
"MR imaging offers excellent anatomical imaging for treatment planning by defining the target volume and organs at risk, [providing] real-time temperature monitoring during therapy, and [allowing] direct evaluation of treatment results," the group wrote.
A dedicated platform
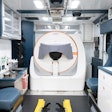
Merckel's group used a dedicated breast platform -- a Sonalleve-based MR-HIFU unit from Philips Healthcare, integrated into that company's Achieva 1.5-tesla MRI scanner -- to partially ablate tumors between 3 cm and 5 cm in women diagnosed with early-stage invasive cancer. The system targeted the breast laterally, reducing the risk that the heart and lungs would receive unintended heat from the procedure. A wide transducer aperture decreased the intensity of the ultrasound energy on the skin. During the treatment, patients were in a prone position on the HIFU table with the targeted breast surrounded by eight 32-element transducers.
Compared with earlier research investigating MR-HIFU for breast cancer ablation, the current study is unique in that the researchers used a dedicated system specifically designed for the application, Merckel told AuntMinnieEurope.com via email.
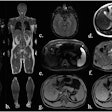
Ten patients with early-stage invasive breast cancer underwent the procedure under sedation before surgery between September 2012 and June 2014. The ablation procedure lasted on average about 2.5 hours. After surgery, Merckel's group assessed the extent of tumors by histopathological analysis.
None of the patients experienced skin redness or burns. Two patients experienced nausea and vomiting in the hours after the treatment, which the researchers attributed to an anesthesia reaction. Eight of the 10 patients reported no pain after tumor ablation while the remaining two reported pain scores of 4 and 5 on a scale of 1 to 10.
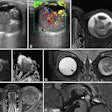
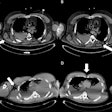
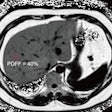
And Merckel's team found a correlation between the applied ultrasound energy of the procedure and tumor necrosis at histopathology: Six of the patients had tumor necrosis ranging from 3 mm to 11 mm after the ablation procedure, and in these patients, the locations targeted by the MR-HIFU procedure was equal to the areas of tumor necrosis.
"[Our] partial ablation approach allowed us to compare viable versus ablated tumor tissue at histopathology," Merckel and colleagues wrote.
Avoiding obstacles
Are there obstacles to the use of MR-guided HIFU in clinical practice? A few, according to Merckel: Determining which patients are eligible for this minimally-invasive treatment may be tricky, and the length of the procedure needs to be reduced.
"Patients with early-stage ductal breast cancer without extensive surrounding DCIS [ductal carcinoma in situ] are good candidates for minimally invasive treatment," she said. To be eligible for MR-HIFU, "the tumor has to be visible on MR, and the distance between the tumor and critical organs has to be greater than 1 cm. And the overall treatment time in this study needs to be reduced to make the technique a clinically attractive alternative for breast-conserving surgery."
In any case, the study represents the next step toward using MR-HIFU for breast cancer ablation, Merckel told AuntMinnieEurope.com. Her team is working on a second study that will investigate the efficacy of breast cancer ablation using this dedicated platform. The upcoming study will include complete tumor ablation rather than partial.
"Our results show that [MR-HIFU] ablation with the dedicated system is safe -- and results in tumor necrosis," she said.