Some 85 billion neurons and upwards of 100 trillion connections -- the adult human brain is the most complex object in the known universe. But how does such a rich neural network grow from a tiny fetus? And how does that growth affect the way our brains ultimately function?
Scientists have a rough picture of how a brain develops in the womb. About three weeks after conception, brain cells begin to form at the tip of the embryo into a tube that will eventually form the spinal cord. This tube then begins to form a brain, where the neurons -- brain cells -- develop and begin to form contacts with each other. Until the fetus is about 20 weeks old, some quarter of a million cells are growing every minute.
However, scientists believe the period from 20 weeks, when the fetus is still in the womb, to 44 weeks, when it is a newborn baby, is critical. This is the period of most rapid change, when all the key connections are made. It may also be when certain connections go wrong, leading to neurological conditions such as autism and cerebral palsy.
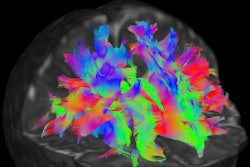
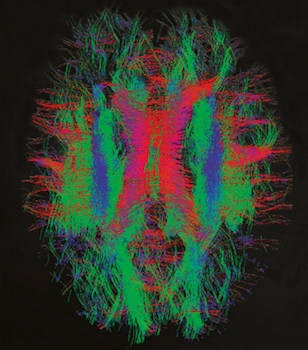 Nerve fiber bundles in a newborn infant's brain. The red tracks are left-right fibers; the green tracks are anterior-posterior fibers; the blue tracks are superior-inferior (top-bottom) fibers. Image courtesy of dHCP.
Nerve fiber bundles in a newborn infant's brain. The red tracks are left-right fibers; the green tracks are anterior-posterior fibers; the blue tracks are superior-inferior (top-bottom) fibers. Image courtesy of dHCP.It is difficult to observe the development in this critical period. Historically, the most direct way to understand brain connections has been to dissect human cadavers. Dissection offers a good way of observing local connections, but it is not so good at revealing longer connections, because the cutting breaks them. Moreover, dissection usually only gives a window into abnormal brains. "We'd like to see the normal state of the brain, too," said Jo Hajnal, PhD, an imaging scientist at King's College London in the U.K.
Hajnal is one of many researchers who are combining their skills in the Developing Human Connectome Project (dHCP), which is using the latest developments in MRI to map brain connections in babies over the critical 20- to 44-week-old period. The project, which will last six years, is being led by King's with support from Imperial College London and the University of Oxford, also in the U.K., as well as global collaborators. It aims to study 1,500 babies before and just after birth. One-third of these babies will have been born normally, another third will be those born prematurely, while the final third will be those who are considered to be at high risk of developing autism.
Ambitious project
It is no small undertaking. Scientists working on dHCP believe it is an ambitious venture, comparing it with early phases of the Human Genome Project, which determined the complete set of human genetic information over 13 years and ended in 2003. "Our view is that no one has ever done this before," Hajnal said. "It's clear that we're on the verge of having the capability to do it, so this will be a bit analogous to the first read of the genome -- a big effort the first time round, in which you get a coarse set of results and a comprehensive view of the situation. Then as time goes on, we'll get better data."
MRI is a time-consuming technique that has until now rarely been performed on unborn babies. When babies are in the womb, their movement is both unpredictable and uncontrollable, which makes it difficult for scientists to get a clear image. Equally problematic is that an unborn baby is, of course, still enveloped by its mother. To get a strong signal in the image, scientists would usually place the machine's receiver coil up against the part of the body under study; for an unborn baby with a womb in the way, this is impossible.
Problems such as these are what Hajnal and others will first have to overcome if they are to successfully image 1,500 born and unborn babies. "That's why I'm involved in the project," he said. "It's a lot of fun."
The precise way in which these problems will be overcome depends on the type of MRI being performed. MRI's basic role is to produce 3D anatomical images, but there are at least two other types of MRI that will prove useful for the researchers involved in dHCP. "An MRI machine is flexible -- you can set it up in lots of different ways," said Timothy Behrens, professor of computational neuroscience in the Oxford group.
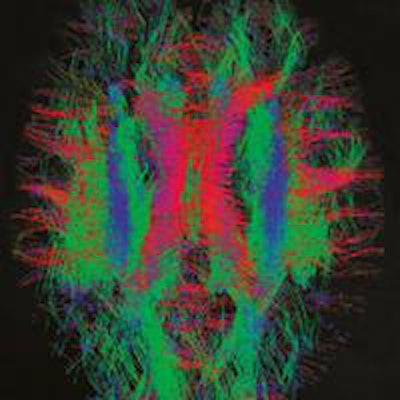
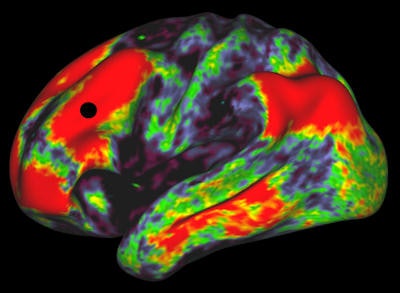 Functional MRI reveals the connection strengths from the black dot to the rest of the brain. This image is from the Human Connectome Project and shows an adult brain. Image courtesy of Human Connectome Project.
Functional MRI reveals the connection strengths from the black dot to the rest of the brain. This image is from the Human Connectome Project and shows an adult brain. Image courtesy of Human Connectome Project.Behrens is developing one of these other techniques: functional MRI, which relies on the fact that hemoglobin -- the red protein found in blood -- has different proton relaxation times depending on whether it is oxygenated or deoxygenated. Functional MRI can be performed very quickly, so that "snapshots" of the brain can be taken every second or so, forming something like a film strip of the movement of blood around the brain.
This is useful because the brain's capillaries, which carry the blood, and the neurons have to work together. If one part of the brain becomes active -- for example if the patient listens to music -- then the neurons in that part will begin to fire. But to do this, the neurons need to be fed with oxygen, and that means a surge in the ratio of oxygenated blood to deoxygenated blood in the nearby capillaries. By detecting this change, scientists can work out where in the brain the activity is happening. Functional MRI also offers a way to map connections, because those parts that are connected will be active at the same time. "Neurological activity and blood flow are intimately linked," Behrens said.
Race to spin
The other MRI technique is diffusion imaging. It is somewhat more complex than the other types, and involves the magnetic field having gradually increasing values over a small region -- in other words, it involves imposing a magnetic-field gradient. The result of this is that the protons will be spinning at different speeds, depending on where they are located.
The best way to imagine diffusion imaging, Behrens said, is as an eight-lane sprint, where it is the lane numbers that tell the participants how fast to run. A certain time after the pistol is fired, the runner (proton) in the first lane will have run much farther (spun more) than the runner in the eighth lane, because the first lane will have made its runner run faster. If all the runners were told to run back, they would all arrive at the start line at the same moment, because the first runner would still be running faster than the eighth to compensate for the extra distance.
But now imagine that the runners are allowed to switch -- or "diffuse" -- between lanes. Having now veered into, say, the third lane, the runner originally in the first lane will run slower, and so will not be able to make the extra distance back to the start line in the same time. Indeed, with all of the runners having switched, the result will be a mismatch in finishing points. Knowing those points, however, it is possible to work out in which lanes the runners started.
The same effect is seen in MRI with a magnetic-field gradient. The value of the magnetic field at a certain point effectively tags the protons with that starting location; so if they diffuse anywhere else, it will still be possible to work out where they started. Diffusion is an important parameter in the dHCP because protons cannot diffuse across membranes in white matter -- the inner part of the brain, through which longer-distance signals are transmitted. By performing diffusion imaging, these white-matter membranes can be mapped.
Part of the way that these three MRI techniques -- anatomical imaging, functional imaging, and diffusion imaging -- can be tailored to unborn and newly born babies is with straightforward practical modifications. For instance, researchers are developing much smaller radioreceiver coils that can be placed close to small babies' heads without discomfort. They will also be using an MRI suite installed within a neonatal critical-care ward, in case any of the babies needs medical attention. "We can do studies on the most delicate babies, while completely encompassing medical cover for them," Hajnal said, who is developing the techniques for in vivo imaging.
Moving targets
Such modifications will not help with the problems of low signal-to-noise nor babies moving around in the womb. Hajnal said that signal-to-noise has largely been improved with recent developments in MRI performance. To avoid fuzzy images produced by movement, his team is working on an alternative MRI technique in which images are taken of just small brain segments at a time. Smaller segments take less time, and hence are less susceptible to blur induced by movement, but they need advanced computer software to piece them back together again.
Once complete, the dHCP will complement the Human Connectome Project (HCP). Led by researchers at Washington University in St Louis, U.S., and with support from the universities of Minnesota in Minneapolis, also in the U.S., and Oxford in the U.K., this project is comprehensively mapping human brain circuitry in 1,200 healthy adults, mostly using MRI. It has been going for three years now with another two years left to run.
dHCP only began in September this year. Currently, its teams are being assembled, and the MRI data-acquisition protocols are being developed and tested. In the next stage, the scientists will begin to take data. The first neuroscience findings are expected to come after 18 months to two years. The scientists are excited about what they will find, but know they have a long way to go. "We've literally just started," Hajnal said.
This article originally appeared in the Physics World Focus on Medical Imaging.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.