Optical coherence tomography (OCT) developer Michelson Diagnostics said that scientists at the University of Leeds in the U.K. have used its VivoSight OCT scanner to develop a quantitative imaging biomarker for scleroderma.
In results published last year in the Annals of the Rheumatic Diseases and highlighted in this month's issue of Nature Reviews Rheumatology, the Leeds researchers led by Dr. Francesco Del Galdo found that OCT of the skin could offer a feasible and reliable quantitative outcome measure in patients with scleroderma, also known as systematic sclerosis.
Scleroderma is a serious autoimmune disorder that affects 1 in 10,000 people and is a progressive disease that involves skin and internal organs. The extent and severity of skin fibrosis is an important prognostic indicator of scleroderma and often serves a primary end point in clinical trials to assess treatment efficacy, Michelson said.
Currently, skin fibrosis is assessed using a clinical semiquantitative method called a "modified Rodnan skin score" based on clinical palpation in 17 body areas using a score from 0 to 3.
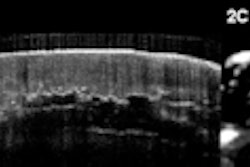
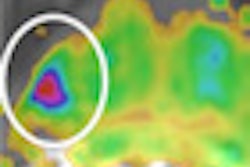
The Leeds group performed 458 OCT scans on 21 systematic sclerosis patients, one morphea, and 22 healthy controls, and compared the results with clinical assessment and histology. They evaluated images captured of the dermal-epidermal junction and dermis using specially designed image processing algorithms, Michelson said.
The significant correlation of the optical density with skin thickness and the excellent inter- and intraobserver reliability of the technique suggest an OCT-based algorithm is an accurate and reliable tool for quantifying skin involvement in scleroderma, the authors said.
The Leeds team is now undertaking a longitudinal study to test the sensitivity of the OCT-based algorithm.