A plethora of clinical trials seeking answers about the value, impact, and effectiveness of lung cancer screening for individuals at risk are under way in Europe. By the end of this decade, the data they generate will help determine the efficacy of making healthcare investments in low-dose CT screening.
Trial outcomes are also expected to produce practical insights into the management of intermediate pulmonary nodules. Interim findings already have reinforced treatment in practice today, according to an article published online 10 August in European Radiology.
Recently published interim results of the North American National Lung Screening Trial (NLST), the largest of the trials with more than 53,000 participants, showed that patients who underwent low-dose CT (LDCT) screening had 20.3% fewer deaths. The NLST compared the effectiveness of LDCT screening versus chest x-ray screening. Both procedures produced high false-positive rates: 95% to 98% for LDCT screening and 93% to 96% for chest x-ray screening. Positive findings were defined as noncalcified nodules 4 cm or larger in the greatest transverse dimension, or suspicious morphology.
With one exception, the nine European trials compare LDCT screening with no screening. Only the French trial Depiscan, with the smallest patient cohort of 765 participants, compared LDCT versus chest x-ray screening.
The European screening trials, of which three are still under way, range in size from the low thousands to almost 16,000 participants in the Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON) trial. The NELSON trial used population-based criteria; the Italian DANTE trial exclusively enrolled at-risk men. The number of rounds of screening and the years when they are performed differ slightly, as did patient follow-up, a range of five to 10 years.
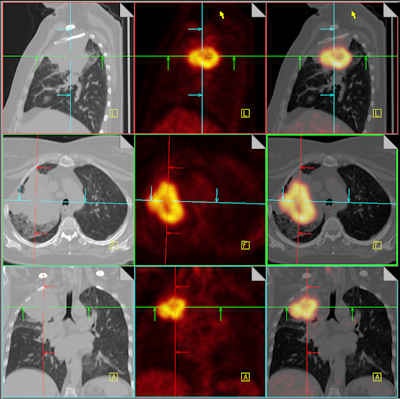 |
| Combining CT with PET adds value for staging non-small cell lung cancer and for monitoring the effectiveness of that treatment. These fused PET/CT images differentiate a large right upper lobe cancer from surrounding atelectasis. Image courtesy of Dr. Nigel Howarth, Institute of Radiology, Clinique des Grangettes, Geneva, Switzerland. Previously published in ECR Today, 5 March 2011. |
It's too early to know the effect of LDCT screening on mortality among diverse populations and to what extent screening may be more cost-effective than other healthcare treatment alternatives for the populations of a country. However, neither individuals nor government economies can afford the burden of acting upon every suspicious nodule identified.
But preliminary results are reinforcing existing pulmonary nodule management concepts, according to the London-based authors Dr. Arjun Nair, from the department of radiology at St. George's Hospital, and Dr. David Hansell, from the department of radiology at Royal Brompton Hospital. The authors write that the newer trials have reinforced current thinking with respect to several interdependent questions. These include defining the characteristics that reliably distinguish malignant nodes from benign ones, identifying the factors that influence variability in node measurement and detection of node growth, and determining the likelihood that an invasive procedure is necessary.
With respect to nodule size at baseline screening, nodules that trigger more aggressive workup range from 10 mm (NLST and NELSON) to 20 mm (DANTE). A volume greater than 500 mm3 is another trigger criteria of the NELSON trial.
Limited results suggest the majority of nodules smaller than 10 mm are benign. The probability that a nodule will demonstrate growth on diameter alone is small.
The NELSON trial was the first study to incorporate software-calculated volume doubling time of nodules, with growth defined as a percentage volume change of 25% or more. This trial defined significant growth requiring action as a volume doubling time of less than 400 days.
Some of the data of the trials suggest volumetric surveillance of nodules could reduce the frequency of LDCT screening exams as currently recommended by Fleischner Society guidelines. In addition to the NELSON trial, results from the Danish Lung Cancer Screening Trial (DLCST) that began in 2004 and the Multicentric Italian Lung Detection Trial (MILD) that began in 2005, suggest the majority of indeterminate nodules with volumes of 50 to 500 mm3 may be reclassified as negative or benign on the next screening. The NELSON trial also confirmed benign lesions could have significant spurts of growth.
The use of morphological criteria has been reinforced by early findings. Analysts of the NELSON study discovered that solid nodules with a lobulated or spiculated margin and an irregular shape increased the likelihood of being malignant, whereas those that were smooth, pleural-based, juxtavascular, or fissure-attached were not malignant and required follow-up at one year only.
Defining the density of nodules is still being investigated, and there is substantial inter- and intraobserver variation in single- and two-dimensional measurements, especially for nodules less than 10 mm in size. Data suggests nodule growth can be detected with high consistency.
The rate of invasive procedures used among the various nodule management protocols ranges between 0.8% and 7.5%, with approximately one-fourth of invasive procedures identifying benign diseases. And one unnecessary thoracotomy would be performed for every 250 subjects screened for lung cancer with LDCT scans, according to a recent meta-analysis of the trial literature.
"The knowledge gleaned from the screening trials at present serves to reinforce and refine, rather than replace, existing nodule management guidelines," Nair and Hansell wrote.