
Researchers at the University Medical Center Utrecht in the Netherlands have now demonstrated that targeted HIFU ablation of the vessels supplying the fibroid could potentially achieve complete fibroid ablation with reduced treatment times. At the International Symposium on MR-guided Focused Ultrasound, held October 17-19 in Chantilly, VA, Marianne Voogt, PhD, presented the team's initial findings.
"The idea for this research was triggered by an observation during treatment that ablation of a small area can result in total fibroid necrosis," Voogt explained. "In the literature, it was already mentioned that this was probably due to vessel ablation, so we decided to investigate this further."
Effective targeting of the supplying blood vessels calls for detailed visualization of the vessel location. The Utrecht team achieved this via pretreatment T1-weighted contrast-enhanced MR angiography (MRA) using a gadolinium-based contrast agent. This produced a detailed 3D map of the uterine arteries and the feeding segmental branches to the fibroid.
The MRA images were merged with pretreatment T2-weighted MR images in three orthogonal planes, enabling optimal localization of vessels. The researchers created treatment plans that primarily targeted locations where vessels entered the fibroid, with margins constrained by surrounding organs and sensitive structures.
Clinical progress
Voogt and colleagues employed the targeted vessel ablation procedure in two patients. Treatment was delivered using the Sonalleve MR-HIFU (Philips Healthcare, Andover, MA), which performs volumetric heating with feedback based on real-time tissue temperature mapping.
The first patient had four small fibroids and two large fibroids, one of which was already devascularized. Treatment focused on the remaining vascularized large fibroid. Following targeted vessel ablation, the large fibroid exhibited 84% devascularization. The researchers also noted complete devascularization of four small fibroids remote from the heated region.
While the total treated volume was 50 mL, the resulting nonperfused volume (determined immediately after treatment) was 211 mL, 4.2 times higher than expected based solely on the thermal dose distribution. The researchers propose that this could be due to the heat causing vessel constriction, which results in decreased cooling by blood, with the increased temperature inducing thermal coagulation of the vessel wall. This, in turn, induces downstream necrosis of fibroid tissue.
The second patient had two large fibroids. In this case, targeted vessel ablation resulted in 90% and 70% devascularization of the treated fibroids. Here, the truly ablated volume was 122 mL and the resulting nonperfused volume was 282 mL, 2.3 times higher than expected based on the thermal dose distribution.
Both patients recovered quickly and without significant adverse events. A three-month follow-up from one of the patients has been performed. She reported a decrease in fibroid symptoms and her fibroids had all shrunk, with a volume decrease varying from 20% to 50%.
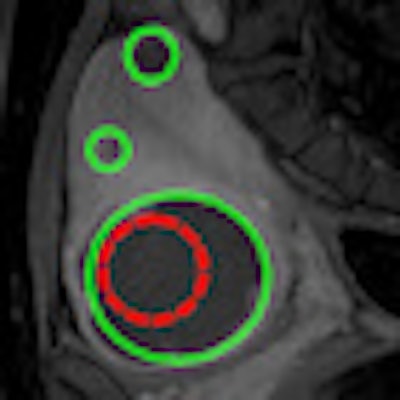
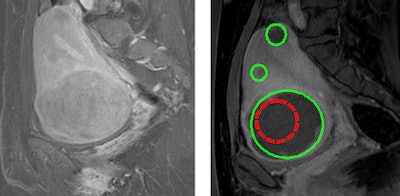 |
| T1-weighted contrast-enhanced MR images before (left) and after (right) treatment. The red region encloses the targeted treated area; the green regions show the nonperfused volume. Images courtesy of University Medical Center Utrecht. |
The researchers observed that some areas containing supplying vessels were more difficult to heat than others. Such regions required longer ablation times and exhibited end temperatures of about 50° C, compared with 60° to 70° C in "normal" fibroid tissue. In the first patient, heating of these more resistant cells also caused pain. They attribute these findings to a heat-sink effect caused by local blood flow.
Another potential limitation of the proposed technique is that planning is currently performed on screening MR images -- as opposed to images taken immediately prior to treatment -- which could result in positioning-related errors. This obstacle could be overcome by integrating the image-processing procedure into the treatment workflow, and the team is currently working to achieve this.
Voogt concluded that targeted vessel ablation may prove promising for increasing the efficacy of MR-HIFU treatment of uterine fibroids and reducing treatment times. She emphasized that visualization of blood vessels supplying the fibroids is essential for effective treatment planning. "We are planning to start a clinical trial for the vessel ablation research, and are awaiting the results of a grant proposal for this subject," she told medicalphysicsweb.
By Tami Freeman
Medicalphysicsweb editor
December 8, 2010
Related Reading
Less invasive options effective for adenomyosis, December 1, 2010
For persistent fibroids, a less invasive option, July 28, 2010
Focused ultrasound burns away uterine fibroids noninvasively, March 17, 2010
Uterine fibroid embolization does not accelerate ovarian reserve decline, January 27, 2010
Laparoscopically assisted cryoablation effective for uterine fibroids, December 29, 2009
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community Web site covering fundamental research and emerging technologies in medical imaging and radiation therapy.