Macrophages, a type of white blood cell, play an important role in the immune system by surrounding and ingesting invading bacteria and other foreign organisms. They are also an integral component of adaptive immunity, attaching themselves to invading antigens and delivering them to other parts of the adaptive immune system to be engulfed and destroyed.
As part of the inflammatory reaction process, however, they are also involved in a number of pathologic processes, including the development of atherosclerotic plaque. Macrophages have been found in high concentrations in unstable atherosclerotic plaques that are at highest risk for plaque rupture.
Meanwhile, the search for a modality that can reliably image the most vulnerable plaque continues. CT, despite its strong capabilities in the detection and measurement of plaque and evaluation of luminal narrowing, has been generally unreliable for assessing plaque composition beyond broad categories of hypodense, dense, or hyperdense calcifications, and even so, Hounsfield unit values can vary significantly depending on factors such as blood flow and contrast timing.
But a new study by Dr. Fabian Hyafil and colleagues from the Mount Sinai School of Medicine in New York City and researchers at the Institut National de la Santé et de la Recherche Médicale and Assistance Publique Hôpitaux de Paris in France may bring intriguing new imaging possibilities to CT by enabling the visualization of macrophages via a nanoparticulate contrast agent administered prior to scanning.
As a result of their animal study, the researchers hope to someday detect the human plaque at highest risk for rupture while there is time to prevent it.
"Macrophages play a key role in acute plaque destabilization and thrombus formation," wrote Hyafil, along with Jean-Christophe Cornily, Jonathan Feig, Ronald Gordon, Esad Vucic, and their colleagues. "They secrete proteases that digest the extracellular matrix and weaken the protective fibrous cap covering the atheromatous core, and they release in atherosclerotic plaques large amounts of tissue factor that accelerate thrombus formation following plaque rupture" (Nature Medicine, April 8, 2007).
The study tested a nanoparticulate contrast agent, N1177. The agent is a suspension composed of crystalline iodinated particles dispersed with a surfactant. The iodinated particles, 90% of which ranged from 153-408 nm in size, were composed of ethyl-3,5-bis(acetylamino)-2,4,6-triiodobenzoate, an estrified derivative of the x-ray contrast agent diatrizoic acid with a very low aqueous solubility, the authors explained. Biocompatible surfactants were added to the particles to prevent aggregation and stabilize their size. The mean iodine concentration of N1177 was 67 mg/mL of iodine.
"Transmission electron microscopy revealed a core consisting of several electron-dense granules covered with molecules of polymer," the group wrote.
To begin, the researchers measured the uptake of N1177 in murine macrophages in vitro; optical microscopy showed dark granules in the cytoplasm of macrophages. "Quantification of iodine in cell pellets, using ICP-MS (inductively coupled plasma mass spectroscopy), confirmed the higher uptake of N1177 by macrophages as compared to a conventional CT contrast agent (4,920 ± 1,019 versus 56 ± 11 mg iodine/g wet weight; p = 0.03)," the team wrote.
Next they performed serial CT imaging of eight nonatherosclerotic New Zealand white rabbits to study enhancement in blood and microphage-rich tissues. For control purposes, the same eight rabbits were imaged a week later using the conventional iodinated contrast agent iopamidol (Isovue, Bracco, Milan) at 250 mg iodine/kg of body weight.
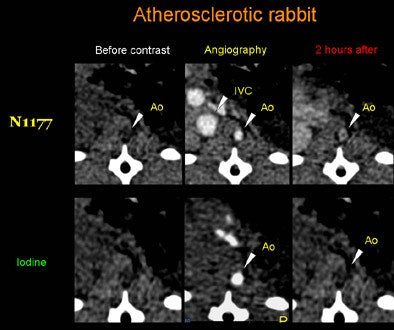 |
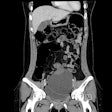
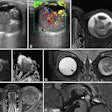
| Axial CT views of the same atherosclerotic plaque of a rabbit before, during, and two hours after the injection of N1177 or a conventional CT contrast agent. Note the strong enhancement of the atherosclerotic plaque two hours after the injection of N1177. All images courtesy of Dr. Fabian Hyafil. |
As hypothesized, the larger size of the particles compared to conventional contrast lengthened the half-life of N1177 in the blood, allowing for delayed arterial and venous angiography, the authors noted. Five minutes postinjection, the blood density of N1177 was 125.3 ± 14.1 HU versus 61.8 ± 13.9 HU with the conventional agent, they reported. Densities in the aortic lumen declined rapidly, and within two hours after injection, the agent had been distributed to organs containing macrophages, including the spleen and liver.
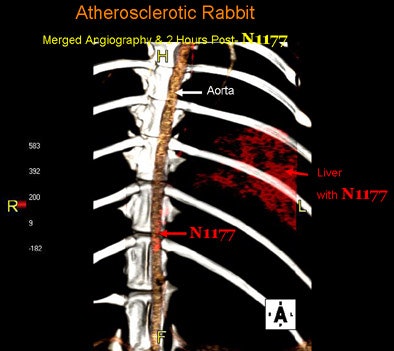 |
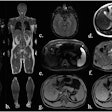
| Fused 3D reconstruction of the aorta of an atherosclerotic rabbit obtained during the injection of N1177 and two hours later with high densities coded using a red color scale. Note the high densities detected in atherosclerotic plaques and the liver two hours after the injection of N1177 allowing for the detection of macrophages. |
The researchers generated atherosclerotic plaques in the eight rabbits by means of a double balloon injury in the aorta and four months of a hypercholesterolemic diet. The rabbits were imaged at CT with the conventional contrast agent, and a week later with N1177. At one to two hours postinjection, enhancement of macrophage-rich tissue was high and the aortic lumen density had returned to baseline levels.
"Hence we were able to discriminate between the enhancement of macrophage-rich atherosclerotic plaques and the density of the aortic lumen in the CT images," Hyafil and colleagues wrote. "Densities measured in atherosclerotic plaques increased from 29.7 ± 6.0 HU before injection to 43.0 ± 7.3 HU two hours after the injection of N1177, compared to 31.5 ± 6.9 HU before and 35.6 ± 8.1 HU after the injection of conventional contrast agent," and N1177 enhancement was significantly higher (13.1 ± 1.0 versus 4.1 ± 0.9 HU, respectively; p < 0.001).
Following the experiments, the animals were killed and the aortas excised for comparison with the imaging results. The specific monoclonal antibody RAM-11, was used for immunostaining. These results showed that plaques with enhancement "higher than 13.3 HU by N1177-enhanced CT showed a macrophage area extending to more than 20% of the intimal area in 90% of the corresponding histological sections," the authors wrote. Immunochemistry detected no macrophage in the aortic wall of control rabbits.
"N1177 provides substantial advantages for the detection of macrophages in atherosclerotic plaques, as compared to previously reported USPIO (ultrasmall superparamagnetic particles of iron oxide)-enhanced MRI or PET imaging with F18 FDG," the group wrote. "It allows for the detection of macrophage-rich tissues with a positive enhancement (that is, increase in signal intensity) as soon as two hours after the intravenous injection of N1177, as compared to the detection with a negative enhancement (that is, decrease in signal intensity) 24 hours after injection of USPIO (assessed using MRI)."
And because CT with N1177 requires no radioactive radiotracer, it "offers higher spatial and temporal resolutions than PET," the researchers stated. "Therefore, N1177 may be useful for the detection of high-risk atherosclerotic plaques, and may become an important adjunct to the clinical evaluation of coronary arteries with CT."
Moving beyond rabbits, phase I studies have already taken place with subcutaneous injection of the contrast agent in human subjects, who did not show any adverse effects, Hyafil wrote in an e-mail to AuntMinnie.com. Phase I studies are planned for the IV administration of the contrast agent in the next two years.
By Eric Barnes
AuntMinnie.com staff writer
May 11, 2007
Related Reading
MDCT correlates ACS to mixed plaques, April 20, 2007
Inflammatory view: Imaging blind to plaque risk, December 7, 2006
Calcium contrast boosts T2-weighted signal in molecular MRI, December 6, 2006
Imaging's big future gets smaller, July 3, 2006
MDCT still can't differentiate between noncalcified plaques, January 10, 2006
Copyright © 2007 AuntMinnie.com