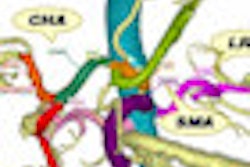
Calypso Medical Technologies of Seattle has signed a nonexclusive product distribution agreement with Siemens Healthcare of Erlangen, Germany, for the sale of its Calypso system in Europe.
Under terms of the agreement, Siemens can sell Calypso's real-time localization tracking technology used for the precise tracking of tumor targets to existing and prospective Siemens linear accelerator customers throughout Europe for a period of three years.
Calypso has received 510(k) clearance by the U.S. Food and Drug Administration (FDA) for use of the system in the prostate and postoperative prostatic bed, and it has received the CE Mark in the European Union.
In 2008, the company entered into a master development agreement with Siemens to jointly develop products integrating Calypso with the Siemens Artiste linear accelerator and other Siemens radiation therapy technologies.
Related Reading
Calypso wraps up first U.K. install, March 17, 2010
Calypso closes $50M funding, September 18, 2009
Calypso, Siemens join forces, September 22, 2008
Copyright © 2010 AuntMinnie.com