In a Swedish study published in the European Journal of Radiology on 12 April, researchers found that the perceived cost-effectiveness of digital breast tomosynthesis (DBT) compared to digital mammography to (DM) is dependent on the relative reduction in breast cancer mortality and patients’ willingness to pay for the screening, as well as future costs of DBT screening exams.
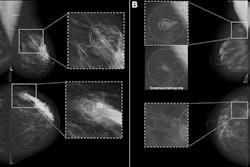
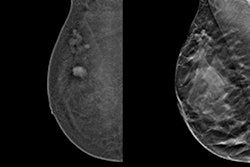
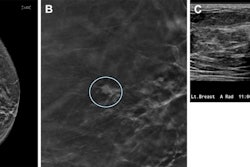
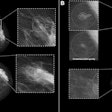
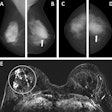
Furthermore, the results of the study by Adam Fridhammar, research manager at the Swedish Institute for Health Economics in Lund, Prof. Sophia Zackrisson, PhD, professor of radiology at the Lund University Breast Cancer Imaging Group, and colleagues showed that while DBT screening detected more invasive cancers than DM, it detected somewhat fewer interval cancers—i.e., those diagnosed after a negative screening but before the next scheduled screening.
Breast cancer screening has a rich history in Sweden, with the first trials being conducted in the late 1970s. In early trials, DM screening led to an estimated 20% reduction in breast cancer mortality rates, with therapeutic advances contributing to further lowering mortality (see breast-cancer-research.biomedcentral.com/articles/10.1186/bcr197). However, since 2023, DBT has been conditionally recommended over DM by the European Commission, particularly for screening women with dense breast tissue, but DBT has yet to make significant inroads in Sweden.
To analyze the cost-effectiveness of instituting DBT screening programs in Sweden, the researchers compared population-based DBT screening to DM screening with a Sweden-specific health economic model using data from the prospective single-arm Malmö Breast Tomosynthesis Screening Trial (MBTST). Women enrolled in the study ranged in age from 40 to 74; input values for the model on demographics, breast cancer incidence, and mortality were derived from regional registries.
As DBT screening is not routine in Sweden, the authors noted, the costs of DBT screening were estimated through expert opinions and information drawn from nine previous studies; the studies used were from the U.S., the Netherlands, Canada, Taiwan, Norway, and Brazil. Four of the studies evaluated DBT as an addition to DM; five assessed DBT only. Three evaluated only women with dense breast tissue while the remainder targeted a population of all women of screening age.
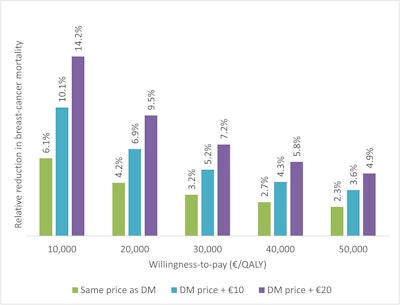 The required relative reduction in breast cancer mortality for DBT-screening to be cost-effective compared to DM-screening, for the same price as DM, DM price + €10, and DM price + €20, at willingness-to-pay thresholds ranging from €10,000 to €50,000/QALY. All figures courtesy of Adam Fridhammar et al and EJR.
The required relative reduction in breast cancer mortality for DBT-screening to be cost-effective compared to DM-screening, for the same price as DM, DM price + €10, and DM price + €20, at willingness-to-pay thresholds ranging from €10,000 to €50,000/QALY. All figures courtesy of Adam Fridhammar et al and EJR.
The average DBT screening cost was calculated at €61. With this average cost, a DBT screening program would increase the current total screening cost by €560,000; the primary treatment costs, by €420,000.
In the team’s analysis, at a calculated willingness-to-pay of between €10,000 and €50,000 (with a cost of exam at a range of €51 to €71), the required relative breast cancer mortality reduction range for cost-effectiveness was from 3.6% to 10.1%, they wrote.
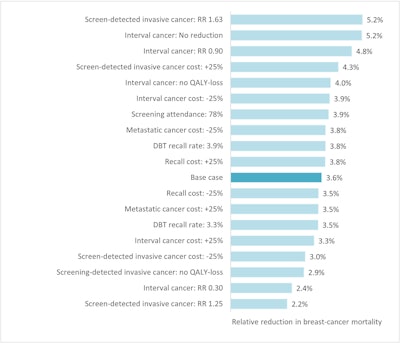 Sensitivity analysis showing the required relative reduction in breast cancer mortality for DBT screening to be cost-effective compared to DM screening, at a cost of €61 for a DBT-screening examination and cost-effectiveness threshold of €50,000/QALY.
Sensitivity analysis showing the required relative reduction in breast cancer mortality for DBT screening to be cost-effective compared to DM screening, at a cost of €61 for a DBT-screening examination and cost-effectiveness threshold of €50,000/QALY.
The research group noted strengths and weaknesses of their assessment. For one, they remarked that it is challenging to compare results of studies and analyses across different healthcare systems with differently designed programs for breast cancer screening and different rates of population risk for breast cancer. Therefore, the results are primarily applicable to countries with similar systems, programs, and risk levels as Sweden. However, the trial only used one round of DBT screening; the increase in detected invasive cancer and/or decrease in interval cancer may not be constant in subsequent rounds of screening, the authors wrote. Moreover, there were few cases of interval cancer, so the rates were unclear. The team also mentioned that factors that could mitigate cost such as AI were not examined in this study.
Read the full EJR study here.