Ultrasound-activated microbubbles can be used to increase the cellular uptake of drugs in vivo, without the need to encapsulate the drugs or otherwise alter their properties, and this enhanced cellular uptake can be tracked using fluorescent dyes. But according to a Dutch study, results can be affected by the rate of the dye's intercellular diffusion and nuclear accumulation, as well as other factors such as dye concentration. This finding may help future investigations into how the uptake-enhancing process works, offering caution against direct comparisons of data from different experimental setups.
The combination of ultrasound and microbubbles facilitates drug uptake via transient permeabilization of the cell membrane. A number of possible mechanisms have been proposed to explain how such a transient permeability is brought about -- including pore formation, and a forced increased in the rate of endocytosis.
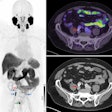
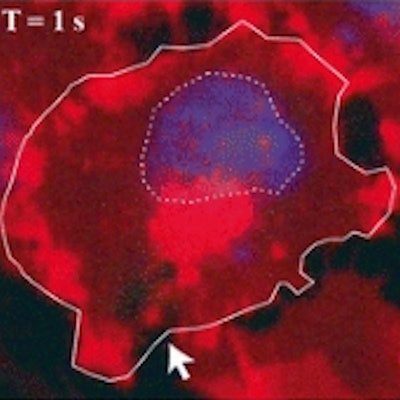
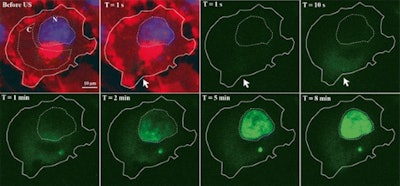 Time series of confocal images, showing cell membrane in red and the nucleus in blue; the white arrow shows the pore created by ultrasound, the SYTOX Green uptake is shown in green. Ultrasound was turned on for 5 second at T = 0. Image courtesy of Bart Lammertink, Clemens Bos, PhD, and colleagues.
Time series of confocal images, showing cell membrane in red and the nucleus in blue; the white arrow shows the pore created by ultrasound, the SYTOX Green uptake is shown in green. Ultrasound was turned on for 5 second at T = 0. Image courtesy of Bart Lammertink, Clemens Bos, PhD, and colleagues.Researchers investigating this process can monitor the uptake of drugs at the individual-cell level using confocal fluorescence microscopy and intercalating fluorescent model drugs, such as SYTOX Green nucleic acid stain or propidium iodide. These compounds, being highly hydrophilic, are not commonly taken up by cells, but when they do, they become highly fluorescent upon binding with nucleic acids, making them a convenient choice for tracking enhanced cellular uptake.
In their new study, however, Bart Lammertink, Clemens Bos, PhD, and colleagues from the University Medical Center Utrecht, in the Netherlands, warn the fluorescence intensity may not be strictly proportional to the cellular concentration of the dyes. "When monitoring the kinetics of cellular internalization, results should be interpreted with caution, as the florescence intensity of these intercalating model drugs not only depends on cellular uptake but also on nucleic acid binding," they caution -- with other experimental parameters also playing a role (Molecular Imaging and Biology, 17 February 2017).
Cell studies
To demonstrate their concerns, the researchers monitored the uptake of various concentrations of SYTOX Green into three different types of cell -- human melanoma, human pharynx squamous carcinoma, and rat glioma cells -- while using either a continuous or pulsed 488 nm laser to excite the dye.
The team found that, upon ultrasound activation, the dye enters the cell and spreads from the entry pore through the cytosol, eventually reaching the nucleus. While the fluorescence intensity in the nucleus continued to increase across the nine-minute observation time, the intensity of in cytosol peaked after 15 seconds -- suggesting that the fluorescence is dependent on the availability of binding sites within the cell, not just uptake alone.
Alongside this outcome, the team also discovered when exposed to a continuous laser, the SYTOX green positive cells were photobleached at a much faster rate -- 6.4 times higher than with a pulsed laser -- as the experiment progressed. A positive correlation was found between the fluorescence kinetics and the concentration of SYTOX green, increasing nearly four times between 1 µM and 20 µM concentration. The choice of cell line was also seen to impact the results, with the rat glioma cells demonstrating a 2.4-fold higher level of fluorescence rate than the human squamous cells in similar conditions.
"The present work demonstrates that the results from investigations of acoustically-mediated membrane permeabilization using fluorescent drug models and optical imaging systems were substantially influenced by experimental parameters," commented Jean-Michel Escoffre, PhD, a biomedical researcher from the Université François Rabelais de Tours in France who was not involved in this study. "Thus, Lammertink and his co-workers have demonstrated that the data from various studies using different fluorescent compounds and/or imaging setups should not be compared directly."
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.