Today's radiologist not only provides images of the highest quality to allow accurate diagnoses, but also must incorporate new biological developments and research into a clinical reality through the use of images. Radiology participates in precision medicine by depicting early abnormalities, predicting prognosis, grading abnormalities, and defining follow-up outcomes, which facilitates clinical decision-making for personalized care. One important feature of precision medicine is the use of virtual biopsies via quantitative imaging evaluation of biologic parameters.1
To be implemented, quantitative imaging approaches must be widely available, reproducible, and standardized in acquisition, signal analysis, and measuring. Before clinical use, biomarkers must be validated in their precision and efficacy regarding the disease process. Imaging biomarkers must be appropriately validated as surrogate end points.2 Biomarkers should be closely coupled to the tissue target disease or treatment effect, their measurements being accurate, reproducible, and feasible over time. Physicians need to know the facts and how they can help to improve early diagnosis, identify the patient phenotype, and select the most appropriate treatment. Implementation of an imaging biomarker has several consecutive steps before it can be used as an innovative information tool in clinical settings.
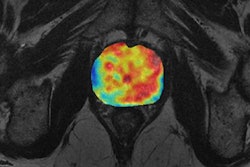
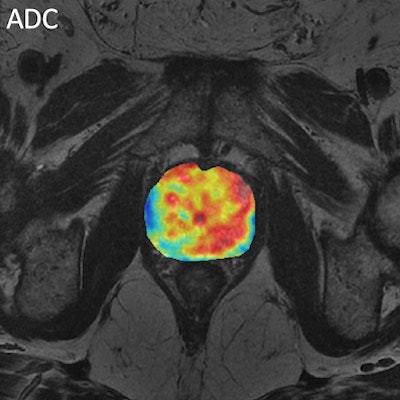
 Figure 1. Multivariate analysis of prostate cancer from MR diffusion and perfusion acquisitions and generation of nosologic map by Euclidean-normalized algorithms.
Figure 1. Multivariate analysis of prostate cancer from MR diffusion and perfusion acquisitions and generation of nosologic map by Euclidean-normalized algorithms.New imaging biomarkers will have an impact in clinical practice only if the information they provide answers the clinical problem. To bring this innovation into clinical practice, biomarker results need to be displayed in an intuitive way. Postprocessing platforms and integrated structured imaging biomarker reports should be implemented and their communication skills tested. The example of prostate cancer might be paradigmatic, where MR can be used either in detection, diagnosis and follow-up evaluation by means of T2, diffusion and perfusion sequences (see figure 1). The proof of concept has to demonstrate that a specific biological hallmark or pathological abnormality might be evaluated using imaging and computational techniques.
Urgent need to monitor validity
The definition of the target hallmark, source images, analytical methodology, and type of measurements are essential aspects that must be considered before studying a specific aspect in a given disease. Integrating an imaging biomarker into clinical practice needs conceptual consistency, technical reproducibility, adequate accuracy, and meaningful appropriateness. The development of a biomarker involves not only the validation of its relationship with the objective reality, but also the monitoring of its overall validity.
New methods and measures have to be validated against the evidence. Defining the gold standards to which the biomarkers have to be compared and the methodologies calibrated is extremely important, and it is the main task of international initiatives like the Quantitative Imaging Biomarkers Alliance (QIBA) from the RSNA and the European Imaging Biomarkers Alliance (EIBALL).3
Traditionally, one of the most referenced methods to validate biomarkers has been the biopsy, but this is an invasive technique, it has sampling biases, and there is a high variability across readers. In comparison with pathology, clinical end points might be more appropriate to validate imaging biomarkers. Measurement uncertainties can be present as there might not be a single right answer in those cases with a heterogeneous distribution of the biological hallmark or a change of the same over time.
As tumors and lesions are nonhomogeneous in their phenotypic, physiologic, components, and genomic aspects, histogram-based analysis of the evaluated parameters may be more appropriate than normal statistical descriptors, such as the mean. Also, the problem of short-term intrinsic variability has to be considered, as in vivo biological examinations might be influenced by physiological changes in the subject and the lesion over time. As an example, liver stiffness for fibrotic evaluation in chronic diseases is subject to the patient fasting state.
Disease outcomes are rarely the result of a single factor entirely encapsulated by one biomarker. Therefore, multivariate, multidimensional, or multiparametric maps might demonstrate the disease's hallmark presence and distribution, in a voxel-by-voxel basis. Parametric images, both conventional and multivariate-nosologic, provide measurements from either the whole tissue being studied (VOI) or only from those areas considered more representative or abnormal (ROI).
Qualification is a measure of the use of a biomarker in specific contents while validation refers to the general performance of the biomarker. Qualification relates to clinical approval while validation relates to the performance of the test. The influence of the center, equipment, technical parameters, and other biases must be scrutinized before the clinical potential can be assessed. Even more, the voxel-measured signal is complex in most clinical situations, as voxel constituents are diverse and might interfere with each other.
Measurements must have a precise relationship (interclass correlation coefficient) with the biological reality. Reducing variability across devices, patients, and time is a must for a reliable and reproducible biomarker. Even more, the clinical usefulness in terms of the benefit for the patients and improvements of outcomes has to be proven.
Industry's role
Some biomedical technological companies, such as QUIBIM, help to evaluate biomarkers via cloud computing and automated pipelines for analysis. Users upload DICOM studies to the platform that integrates, among other features, a zero-footprint viewer. After analysis, the results can be downloaded in readable structured quantitative reports (figure 2).
 Figure 2. Example of structured quantitative reports generated by QUIBIM.
Figure 2. Example of structured quantitative reports generated by QUIBIM.For more information on this area, you may want to attend the European School of Radiology (ESOR) Asklepios Course on Body Imaging Biomarkers, which will be held on 12-13th December 2016 in Valencia, Spain. The meeting has the official endorsement of the ESR. For registration, click here.
Dr. Luis Martí-Bonmatí, PhD, is director of medical imaging at La Fe University and Polytechnic University Hospital, and chief of radiology at Quirón Hospital in Valencia, Spain. He is also professor of radiology at Valencia University.
Dr. Angel Alberich is scientific/technical director of the GIBI230 -- Biomedical Imaging Research Group and CEO of QUIBIM S.L., a reference core lab in imaging biomarkers extraction and analysis. Established in 2012 as a spin-off of La Fe Health Research Institute in Valencia research institute of La Fe Polytechnics and the university hospital, the company derives its name from quantitative imaging biomarkers in medicine. It has collaborative relationships with Spanish hospitals and focuses on the processing and extraction of biomarkers for medical imaging workflows.
The comments and observations expressed herein do not necessarily reflect the opinions of AuntMinnieEurope.com, nor should they be construed as an endorsement or admonishment of any particular vendor, analyst, industry consultant, or consulting group.
References
- Frangi AF, Taylor ZA, Gooya A. Precision imaging: More descriptive, predictive and integrative imaging. Medical Image Analysis. 2016;33:27-32.
- Martí Bonmatí L, Alberich-Bayarri A, García-Martí G, et al. Imaging biomarkers, quantitative imaging, and bioengineering. Radiologia. 2012;54:269-78.
- Martí-Bonmatí L, Alberich-Bayarri A. Imaging Biomarkers: Development and Clinical Integration. Springer; 2017.