Biomarkers, in combination with liquid biopsy and pathology, can help to optimize cancer detection and characterization in the years ahead, and they now form an integral part of a diagnostic approach that aims to integrate diverse data from imaging, pathology, and liquid biopsy/laboratory medicine, noted experts at this morning's New Horizons session (NH 1, "The role of imaging in the era of liquid biopsy").
"There's a movement now that says diagnosis is optimized by the integration of all three," Dr. Heinz-Peter Schlemmer, director of the department of radiology and coordinator of the imaging and radio-oncology research at the German Cancer Research Centre (DKFZ) in Heidelberg, Germany, told ECR Today. "For a long time, we've been seeking quantitative data from imaging that lets us detect a cancer early and determine tumor aggressiveness, local infiltration, and metastatic pattern and to plan and monitor therapies."
Combining liquid biopsy and imaging looks set to improve future surveillance by detecting subclinical disease earlier and, ultimately, will deliver better outcomes, according to Dr. Vicky Goh, chair of clinical cancer imaging at King's College Hospital and a consultant radiologist at Guy's and St Thomas' Hospital in London.
"By accurately quantifying disease burden and localizing disease sites, it is possible currently to improve patient selection for further therapy," she said. "Targeted molecular imaging will have an increasing role, particularly as disease is detected earlier and its burden is reduced."
In the era of liquid biopsy, there will still be an important role for imaging and a need for greater sensitivity. In this respect, Goh stressed the need for more sensitive imaging. This morning, she explained how cancer surveillance aims to detect disease progression at an early enough stage for further definitive treatment to be a success.
Turning to his main interest, Schlemmer noted that, so far in clinical studies, morphology acts as a biomarker quantifying tumor size. During therapy, it can show whether the tumor is regressing, progressing, or stable, thus indicating whether a treatment is effective.
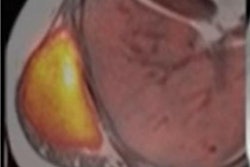
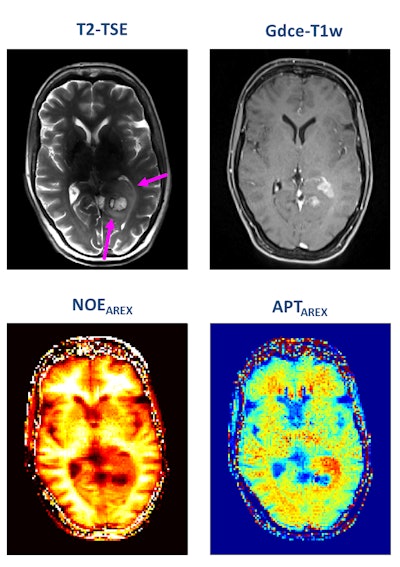 A patient with newly diagnosed glioblastoma underwent relaxation-compensated chemical exchange saturation transfer (CEST) imaging at 7.0 tesla. The protein-weighted CEST contrasts by means of amide proton transfer (APT) and nuclear Overhauser effect (NOE). MRI shows distinct alterations of protein concentrations in the tumor area. While APT signals are markedly increased, NOE-mediated CEST effects drop in the tumor area. The endogenous CEST contrasts have recently proven high potential as novel MR biomarkers to noninvasively assess tumor characteristics and prognosis. (See: Paech D, Windschuh J, Oberhollenzer J, et al, Assessing the predictability of IDH mutation and MGMT methylation status in glioma patients using relaxation-compensated multi-pool CEST MRI at 7.0 T, Neuro-Oncology, November 2018, Vol. 20:12, pp. 1661-1671, and Regnery S, Adeberg S, Dreher C, et al, Chemical exchange saturation transfer MRI serves as predictor of early progression in glioblastoma patients, Oncotarget, June 2018, Vol. 9:47, pp. 28772-28783.) Image courtesy of Dr. Heinz-Peter Schlemmer.
A patient with newly diagnosed glioblastoma underwent relaxation-compensated chemical exchange saturation transfer (CEST) imaging at 7.0 tesla. The protein-weighted CEST contrasts by means of amide proton transfer (APT) and nuclear Overhauser effect (NOE). MRI shows distinct alterations of protein concentrations in the tumor area. While APT signals are markedly increased, NOE-mediated CEST effects drop in the tumor area. The endogenous CEST contrasts have recently proven high potential as novel MR biomarkers to noninvasively assess tumor characteristics and prognosis. (See: Paech D, Windschuh J, Oberhollenzer J, et al, Assessing the predictability of IDH mutation and MGMT methylation status in glioma patients using relaxation-compensated multi-pool CEST MRI at 7.0 T, Neuro-Oncology, November 2018, Vol. 20:12, pp. 1661-1671, and Regnery S, Adeberg S, Dreher C, et al, Chemical exchange saturation transfer MRI serves as predictor of early progression in glioblastoma patients, Oncotarget, June 2018, Vol. 9:47, pp. 28772-28783.) Image courtesy of Dr. Heinz-Peter Schlemmer.Numerous other biomarkers, many of which are functional, are associated with tumors, and these can be quantified with MRI. He pointed out that certain functional biomarkers are readily available, but they have not yet been standardized, and this limits their application at present. Quantification of lesion perfusion over time is a case in point.
"Tumors are usually hyperperfused with neovascularization, and this can be detected very early, long before the tumor size shrinks," said Schlemmer, adding that there are different methods for measuring perfusion by MRI.
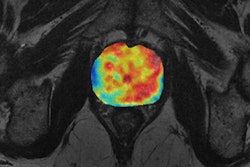
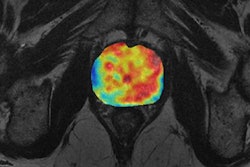
Other functional biomarkers that are typical cancer hallmarks include diffusion-weighted imaging (DWI) with MRI, which looks at cell density and composition of the extracellular matrix. DWI is an established technique for diagnosing prostate cancer and is starting to be used with melanoma too.
Schlemmer explained that cancer drugs and radiotherapy have an impact on cell density because as the cells die, so the extracellular space widens. The effect is seen on MRI early in treatment. With chemotherapy, these diffusion changes can happen within a couple of hours of starting therapy, and they are a powerful indicator measuring the depth of tumor cells.
Referring to the overarching aims of using imaging biomarkers, he said that it was important to both detect tumors in the first instance but also to determine if a tumor is responding to therapy.
"Different mass lesions seen on morphological images do not indicate the malignant potential of the lesion. But with diffusion imaging, we can see increased cellular density in the mass lesion, and, in addition, with dynamic contrast perfusion imaging, we can see neovascularization, providing a strong indication that this mass lesion belongs to a malignant tumor," explained Schlemmer, adding that diffusion and perfusion are known biological cancer hallmarks, reflected by imaging biomarkers.
These are the most important radiological biomarkers in clinical use. Although these biomarkers have been available for a few years, radiologists still struggle to use them effectively in clinical trials because there has been no standardization between different machines and operators, according to Schlemmer, whose team is working toward improving this.
Turning to some of the newer imaging methods, he mentioned that chemical exchange saturation transfer (CEST) imaging is showing promise because it allows for the measurement of protein content in the tumor.
"Change in protein content is important. We don't fully understand the biological significance of this, but we have seen meaningful changes, particularly in brain tumors," he noted. "We've also seen a genetic mutation that results in a measureable difference in protein content. We hope that in time we will use CEST for other applications."
There are various further new imaging biomarkers. One technique, for example, is MR fingerprinting, which involves sequences that randomly acquire data and the output is correlated with a biological biomarker. "We don't fully understand how it works but it does," he commented.
Looking ahead, Schlemmer turned to artificial intelligence and postprocessing as used in imaging. "We can use deep learning for extracting information out of images that are not visible to the eye, not even to a highly trained radiologist," he said. "Deep learning can pick up on this in a useful quantitative way that provides information that is easily transferable between hospitals."
He described a deep-learning method for detecting breast cancers that he and his colleagues have tested. After carrying out DWI in a screening population, the data were read by a deep-learning programme and then read by a radiologist. "We found that a computer can evaluate the data as well as an experienced, well-trained radiologist can, so even though there is no gain in knowledge, a less experienced radiologist could use the process and the quality of reading and reporting can be increased," said Schlemmer, adding that his team has also used deep learning with prostate cancer.
Originally published in ECR Today on 27 February 2019.
Copyright © 2019 European Society of Radiology