A handheld optoacoustic scanner enables imaging of skin layers and blood vessels in patients with psoriasis. Developed by researchers from Helmholtz Zentrum München and the Technical University of Munich (TUM), the device uses raster scan optoacoustic mesoscopy (RSOM) to provide clinically relevant information, without requiring contrast agents or radiation exposure.
Instead, RSOM uses a weak laser pulse to excite the tissue of interest, which then absorbs energy and heats up slightly. This heating causes momentary tissue expansion, which generates ultrasound waves that can be used to reconstruct a high-resolution image of structures under the skin, according to an article posted online in Nature Biomedical Engineering (10 May 2017).
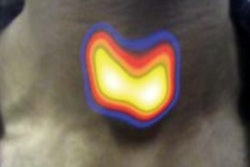
 A newly developed tissue scanner allows visualization of the structure of skin layers and blood vessels under the skin of psoriasis patients. Image courtesy of Helmholtz Zentrum München.
A newly developed tissue scanner allows visualization of the structure of skin layers and blood vessels under the skin of psoriasis patients. Image courtesy of Helmholtz Zentrum München.The researchers demonstrated RSOM's performance by examining cutaneous and subcutaneous tissue from psoriasis patients. RSOM provided measures of skin thickness, capillary density, number of vessels, and total blood volume in the skin, which the researchers used to define a novel clinical index for assessing psoriasis severity. In future, they plan to use RSOM to assess other diseases such as skin cancer or diabetes.
"This technology, which is easy to use, is allowing us to acquire the first new insights into disease mechanisms. It also facilitates treatment decisions for the physicians," said Dr. Vasilis Ntziachristos, director of the Institute of Biological and Medical Imaging (IBMI) at the Helmholtz Zentrum München and chair of biological imaging at TUM.
Algorithm improves angiogenesis detection
In other news, researchers at the digital research network Data61 are developing a software tool to improve detection of angiogenesis and help provide early diagnosis of malignant tumors.
Teaming up with scientists at the Shanghai Institute of Applied Physics at the Chinese Academy of Sciences the Data61 team analyzed 26 high-resolution 3D micro-CT images (produced by the Shanghai Synchrotron Radiation Facility) of the brains and livers of mice with various stages of cancer.
From these images, they developed a robust algorithm to generate an accurate representation of the vasculature, using a technique called end-point constraints. End points are critical in preserving the geometrical features of new blood vessels. The software allowed the researchers to measure subtle changes in blood vessel proliferation, including the number and length of vessel branches, and produced significantly clearer skeletons of the vasculature than previously possible.
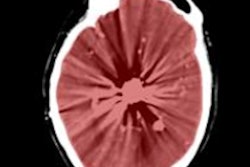
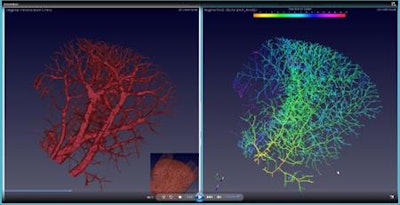 Comparison of the enhanced blood vessel prior to (left) and after the end-point skeletonization process (right).
Comparison of the enhanced blood vessel prior to (left) and after the end-point skeletonization process (right).The synchrotron beamline used in this study generates radiation levels unsafe for human imaging. To progress to clinical trials, the team is partnering with a hardware manufacturer to produce high-resolution images with safe radiation levels.
"Our robust algorithms for the early detection and quantification of angiogenesis could potentially be a great step forward in the detection and treatment of cancer," said Dr. Dadong Wang, the leader of the CSIRO Quantitative Imaging Research Team, part of the CSIRO Data61 project. "They can also be applied to a wide range of other applications, such as analysis of 3D neurite outgrowth for drug development. While there is great interest in taking these findings further, there is still a long way to go before this new development can be applied to human patients. But we are very hopeful, and currently looking for collaborators and partners to take the technology to the next stage."
Multicolor in vivo imaging
SPECT and PET are the two most common molecular imaging techniques, but radioactive tracers suitable for each detector are limited in terms of energy. The use of a Compton camera, which can image gamma rays with energies from a few hundred keV to more than MeV is eagerly awaited, along with the development of potential new tracers.
With this in mind, a team at Waseda University from Shinjuku, Tokyo, has designed a compact Compton camera that weighs just 580 grams and fits in the palm of a hand. The researchers used the device to perform the first multicolor 3D molecular in vivo imaging of a live mouse administered with three tracers: iodine-131 (I-131; 364 keV), strontium-85 (Sr-85; 514 keV) and zinc-65 (Zn-65; 1116 keV). Rotating the camera around the mouse enabled simultaneous in vivo imaging of the multiple tracers in nearly real-time with a resolution of 3 mm. Tri-colour gamma-ray fusion images demonstrated that I-131, Sr-85, and Zn-65 are effective new tracers that accumulate in the thyroid, bones, and liver, respectively (Scientific Reports, 18 May 2017).
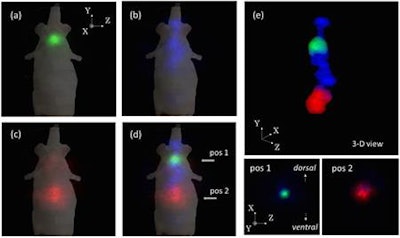 Representative 2D slice in the Z-Y plane of a 3D image of a mouse: (a) I-131, (b) Sr-85, (c) Zn-65, and (d) fused images of all three tracers and (e) the 3D image and the tomographic images of the fused image in the Z-X plane at the two indicated positions. Images courtesy of Waseda University.
Representative 2D slice in the Z-Y plane of a 3D image of a mouse: (a) I-131, (b) Sr-85, (c) Zn-65, and (d) fused images of all three tracers and (e) the 3D image and the tomographic images of the fused image in the Z-X plane at the two indicated positions. Images courtesy of Waseda University."The measurement took 10 minutes per angle, so we were able to obtain an image taken from 12 angles in just two hours. The time could be reduced even more by using multiple Compton cameras," explained group leader Jun Kataoka, PhD. "For example, if there are 12 Compton cameras surrounding an object, the same image as this study could be obtained in just 10 minutes, suggesting a new way to understand biodynamics by looking at how a drug is taken into the body in 10-minute increments."
Based on this study, Kataoka is now developing a gamma-ray camera that works like the human eye: "The human eye can instantly distinguish the colors and brightness of light from all directions, as well as determine the object's shape in 3D from the displacement between the left and right eye. Therefore, stereoscopic imaging becomes theoretically feasible by using multiple ultracompact Compton cameras," he said.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.