What imaging modality should a physician use to treat a patient after a stroke? The answer is complicated, but a Swedish-Singaporean team sought to shed light on this conundrum in a study published on 14 February in the European Journal of Radiology.
Dr. Leonard Yeo and colleagues presented some of the current techniques in use to image stroke -- and others still in the realm of research -- that have not yet transitioned into clinical practice.
"The practical challenge for physicians who treat stroke is to evaluate the pros and cons of each technique and select the best choice for the situation," wrote Yeo, who is from the neurology division at the National University Health System in Singapore. He is also an interventional fellow working on his doctorate in the neuroradiology department at Karolinska University Hospital in Stockholm.
"This is no simple task, and there are many factors to consider, including the differential diagnosis, which needs to be evaluated, the availability and reliability of the imaging technique, and time and expertise required to perform and interpret the scanning," he and his colleagues wrote.
The financial cost of the modality, patient monitoring during the imaging procedure, and patient comfort also need to be considered, they noted.
Why try something new?
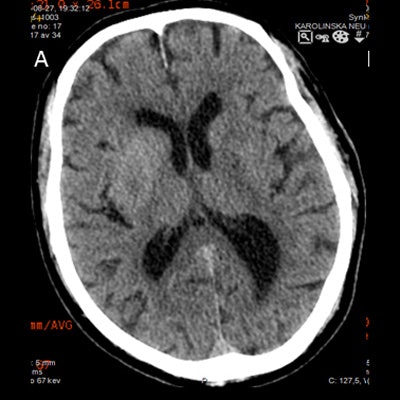
The role of imaging after treating stroke is not well-defined, but it is important because imaging detects and monitors hemorrhagic complications, especially intracranial hemorrhage -- the most feared complication. A routine noncontrast CT brain scan 24 hours after treatment is recommended as part of stroke guidelines. This confirms the initial stroke diagnosis and provides prognostic information.
However, the benefit of performing noncontrast CT scans in asymptomatic stable patients remains contentious with contradicting studies. Thus, repeat imaging should be judiciously applied with clinical judgment, according to the authors.
"In Singapore and Karolinska, the standard postischemic imaging is plain CT, preferably with dual-energy technique; however, this is tempered by the clinical situation," Yeo wrote in an email to AuntMinnieEurope.com. "In more complex cases, other imaging modalities such as MRI or even a perfusion scan may be warranted."
Noncontrast CT
Noncontrast CT is the cheapest, quickest, most widely available, and easiest method for detecting intracerebral hemorrhage after an ischemic stroke, although MRI also comes close with appropriate sequences, the authors noted.
Noncontrast CT can detect hyperdense areas due to either hemorrhage, extravasation (enhancement) of contrast medium, or disruption of the blood-brain barrier. For these lesions, serial imaging is necessary for a concrete diagnosis to distinguish gradual contrast material extravasation from an increase of hyperdensities due to hemorrhage, they added.
Approximately 29% to 50% of hyperdense lesions are secondary to contrast extravasation, defined as a lesion with less than 90 HU persisting on a 24-hour noncontrast CT scan, and may be associated with parenchymal hematoma and unfavorable clinical outcomes. Also, some evidence suggests patients with higher Hounsfield units are more likely to have significant hemorrhage.
Also, follow-up CT angiography (CTA) can be performed in less than a minute in conjunction with follow-up noncontrast CT. It could have a role in estimating functional outcomes after stroke treatment.
"It proves that the previously occluded arterial segment remains fully open, and the source images from the CTA can provide a qualitative cerebral blood volume map to estimate the infarction core, which may improve the calculation of the brain at risk to become the final infarcted area compared with just a noncontrast CT," they added.
Mandatory postprocedure noncontrast CT is performed at many institutions, but it requires transport of the patient from the angiography suite to the CT scanner, costing time, affecting safety, and increasing costs.
Flat-panel CT
Flat-panel CT can avoid some of the pitfalls of noncontrast CT, because flat-panel fluoroscopic detectors opportunistically scan fluoroscopic images into the same format as traditional CT scanners. Postprocedural flat-panel CT can detect intracranial hemorrhage with 91% to 100% sensitivity and negative predictive value (NPV), but it has only 88% specificity and 44% to 84.2% positive predictive value. Also, it has inferior image quality in the posterior fossa, according to the authors.
"While flat-panel CT is currently not reliable in depicting ischemic brain lesions, it appears to be an effective tool for immediate identification of the postprocedural intracranial hemorrhage with good interobserver agreement and sensitivity similar to or better than what has been reported in the literature with noncontrast CT," they wrote.
Also, the high NPV of flat-panel CT may preclude the need for 24-hour follow-up CT or MRI. Flat-panel CT has also been evaluated for procedural complications such as arterial perforations. However, it has limits in terms of spatial resolution -- flat-panel CT can't accurately differentiate between hyperdensities due to the rupture of the blood-brain barrier or a small iatrogenic perforation.
Another area in which noncontrast CT falls short is distinguishing between extravasation from a disruption of the blood-brain barrier and intracranial hemorrhage.
Dual-energy CT
Using dual-energy CT (DECT), characterization of a disruption of the blood-brain barrier and hemorrhage becomes possible using varying attenuation characteristics, which have been activated by two particular energy levels, thereby allowing DECT to differentiate between contrast extravasation and intracranial hemorrhage. Iodine overlay maps can be reconstructed, with a corresponding virtual unenhanced noncontrast image.
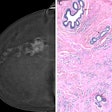
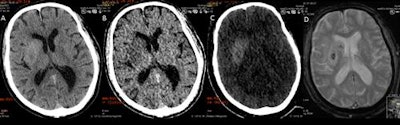 DECT can suggest only limited breakdown of the blood-brain barrier, while the MRI gradient echo reveals the true hemorrhage. A: virtual polychromatic CT. B: water-weighted. C: iodine-weighted. D: Gradient-echo sequence of MRI. Image courtesy of Dr. Leonard Yeo.
DECT can suggest only limited breakdown of the blood-brain barrier, while the MRI gradient echo reveals the true hemorrhage. A: virtual polychromatic CT. B: water-weighted. C: iodine-weighted. D: Gradient-echo sequence of MRI. Image courtesy of Dr. Leonard Yeo.Hyperdensity seen on mixed images and persisting on virtual unenhanced noncontrast images but not on iodine overlay maps is considered a hemorrhage, while a hyperdensity present on mixed images and iodine overlay maps but not on virtual unenhanced noncontrast is considered contrast extravasation.
DECT has an accuracy of 89% in this regard. Routine use of the modality allows for reliable distinction between disruption of the blood-brain barrier and intracranial hemorrhage just after endovascular stroke therapy, so the treating physician can allocate resources such as intensive monitoring and further therapy, according to the authors.
"Further, it may guide the physician when to start secondary prophylaxis with antiaggregation, as contrast extravasation probably poses a much lesser risk for a significant hemorrhagic conversion than presence of intraparenchymal blood," they wrote.
Still, despite its advantages, DECT is not able to increase the accuracy of ischemic diagnosis, and a study showed disruption of the blood-brain barrier on DECT was not an accurate predictor for final infarct size.
Techniques currently not in use
In addition to CT techniques used in clinical practice, Yeo and colleagues also discuss modalities still in the preclinical phase, such as diffusion-tensor imaging (DTI). DTI is an MRI technique that allows for visualization of white-matter tracts and may have a prognostic value in acute stroke, especially when the ischemic areas transact important tracts.
Corticospinal tract integrity can also be assessed in conjunction with diffusion-weighted imaging (DWI) to predict the probability of motor recovery after a corona radiata stroke. DWI is also often used to detect emboli related to endovascular procedures, but it has some limitations. For instance, a large initial DWI lesion can mask secondary treatment-related emboli when they overlap.
A method that doesn't have such drawbacks is susceptibility-weighted imaging (SWI), an MRI sequence used to detect both thrombi and hemorrhage on follow-up scans and that has better sensitivity than DWI.
"Most emboli have an elongated shape and are parallel to the vessels, while microbleeds are typically round in shape, which allow SWI to differentiate between emboli and hemorrhagic transformation," the authors noted.
It could be a valuable complement to assess the success and safety of endovascular procedures, and its ability to noninvasively visualize emboli makes SWI an interesting tool to evaluate the quality of novel thrombectomy techniques and instruments, according to Yeo and colleagues.
They are currently looking at the way the leptomeningeal collateral circulation evolves during the acute period after stroke and how it can be used to predict outcomes. They are also developing new devices in cooperation with several companies to optimize the thrombectomy procedure.