In a comparison of PET images with three different radiotracers, German researchers found that tau deposits may be more influential in causing Alzheimer's disease than amyloid accumulation, according to results presented at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) meeting.
The research team led by Gerard Nisal Bischof, PhD, from the University Hospital of Cologne reported a significant correlation between increased tau and decreased metabolic activity in the brain. Such hypometabolism is an indication of neurodegeneration, according to the group. In addition, the results demonstrated the utility of AV-1451, a novel PET tracer that binds to tau in the brain.
"Our results emphasize the utility of in vivo tau imaging to further understanding of the disease mechanism of Alzheimer's," Bischof told SNMMI attendees. "Our findings also corroborate the view that tau pathology is more intimately linked to the neurodegenerative aspect of the Alzheimer's disease process."
Alzheimer's damage
More than 46 million people worldwide are estimated to have Alzheimer's disease, and more than 130 million people are expected to be afflicted by 2050. The global economic cost of the disease also could reach $1 trillion by the middle of this century, according to Alzheimer's Disease International.
Previous research has linked Alzheimer's disease and other neurodegenerative diseases with beta-amyloid plaques and neurofibrillary tangles of tau proteins in regions of the brain that handle cognitive and executive functions. Much of the research has been conducted postmortem, which does not contribute any significant insight into how the disease develops, especially early in a person's lifetime. In addition, the potential effect of both amyloid and tau proteins on neurodegeneration remains unconfirmed.
"A better understanding of the relative contribution of tau and beta-amyloid pathology to neurodegeneration in Alzheimer's will advance our knowledge of disease mechanisms and provide crucial information for disease-modifying interventions," the authors wrote in their SNMMI abstract.
For the study, Bischof and colleagues enrolled 10 patients with a mean age of 67.2 years (± 7.3 years). They used FDG to evaluate glucose hypometabolism and neuronal dysfunction, the tracer AV-1451 to measure tau deposits, and Pittsburgh Compound B (PiB) to detect amyloid plaques. The study also included 50 healthy control subjects with an average age of 64 years for comparison.
Hypometabolism factors
The researchers found that increases in tau protein were directly associated with hypometabolism in the parietal, temporal, and occipital lobes, while there was no strong association between amyloid accumulation and hypometabolism. However, there was a connection between tau and amyloid in the parietal cortex: Regional tau deposition had a stronger influence on metabolic activity in regions with greater amyloid burden.
"The strong indication of both protein pathologies to hypometabolism in parietal regions is in line with its regional susceptibility where tau and amyloid may promulgate their effect," Bischof said.
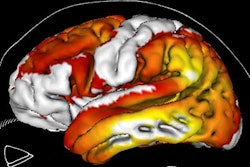
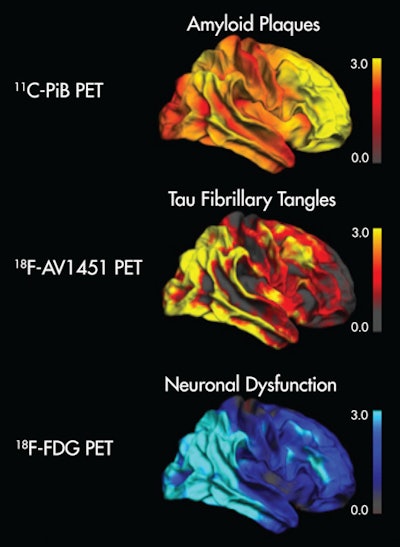 Image shows the topographical correspondence of tau but not amyloid pathology with neuronal dysfunction in Alzheimer's disease. The right lateral surface of projected z-score images reflects deviation from healthy controls. Yellow/red indicate higher uptake, while blue denotes lower uptake compared to controls. Image courtesy of Bischof et al and SNMMI.
Image shows the topographical correspondence of tau but not amyloid pathology with neuronal dysfunction in Alzheimer's disease. The right lateral surface of projected z-score images reflects deviation from healthy controls. Yellow/red indicate higher uptake, while blue denotes lower uptake compared to controls. Image courtesy of Bischof et al and SNMMI.The researchers suggested that tau imaging appears closely linked to the actual onset of neuronal damage, while amyloid imaging may allow clinicians to detect a predisposition to disease many years ahead of the onset of symptoms.
Bischof and colleagues recommended additional research into these findings and the causes of neurodegeneration in living dementia patients, which could improve diagnostic accuracy and lead to new drugs that could potentially target tau to slow or stop degenerative conditions in the brain.
Such multimodal imaging approaches could provide more precise staging of neuropathology, even before the onset of memory loss experienced by Alzheimer's patients, according to the authors. Furthermore, improved prediction, prognosis, and therapy control and follow-up may become possible.