Rigorous planning and continuous observation of the process are essential to achieve the migration of old DICOM data into a new PACS in a reasonable time frame, and a proper test migration is necessary to eliminate potential pitfalls, according to a leading European expert. But with any migration, users must take a limited amount of risk because not all problems can be identified.
"PACS migration is a very difficult and challenging process," noted Peter van Ooijen, PhD, from the radiology department at the University of Groningen in the Netherlands. "In the PACS transition project, the data migration from the previous PACS to the new one is the most challenging task. To successfully perform this task, careful planning is required."
Before starting the process, he thinks these key questions must be addressed when planning a transition to a new PACS environment: What are the prerequisites of the current PACS to allow successful migration? What migration steps and methodologies should be adopted and followed? What are the possible problems and challenges? What might occur, and what is the possible solution? How will the reporting and tracking of issues encountered during the process be implemented?
In his e-poster presentation at ECR 2014, van Ooijen set out to inform congress delegates about the issues involved in a migration project for PACS transition and to help in planning such a migration. He presented a project planning structure, together with an overview of the possible problems that can occur during the migration of image data.
"Migration requires the full cooperation of the current and future PACS vendors. Furthermore, compliance to the DICOM standard is essential for an easy transition," he emphasized. "Operations like C-FIND, C-MOVE, and C-STORE should be supported to deliver original, correct, DICOM files. Another important factor in the migration is the requested availability of the legacy PACS and its capability to both run a full department and a very demanding migration at the same time. Careful planning together with the legacy PACS vendor is advised to obtain optimal windows during which the migration can take place."
The whole migration process should be thoroughly investigated in terms of the required time, manpower, and equipment resources, he added. A standard setup using a dedicated migration tool can be used to retrieve nonpixel data (metadata) from the old PACS and validate the nonpixel data with the content of the radiology information system (RIS).
After validation, correct studies are sent to the new PACS by instructing the old PACS to perform "a DICOM send" of those studies; incorrect data are registered and can be transferred to a so-called dead-letter file to allow missing studies to be searched for at a later date.
Furthermore, the RIS can be used to retrieve reports for inclusion in the new PACS environment, and within the migration tool, rule-based exclusion can be configured to transfer certain data to trash (e.g., test patient and research-related data), stated van Ooijen.
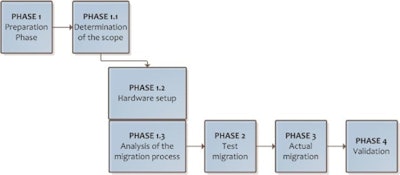 The main steps should be defined in the migration protocol. Source: Peter van Ooijen, PhD, University of Groningen, presented at ECR 2014.
The main steps should be defined in the migration protocol. Source: Peter van Ooijen, PhD, University of Groningen, presented at ECR 2014.The preparation phase should determine the scope of the migration, the hardware setup and analysis of the process resulting in a sound migration plan. A test migration should be performed to identify possible problems that can be solved before the actual migration is started.
Also, the point of switching from the viewing workstations of one vendor to the other should be included with clearly defined go/no go decisions, he explained.
"In the validation phase, a final evaluation of the validity of the data in the new PACS environment should be performed to determine correctness and completeness of the data received and to provide final acceptance of the data cleanup and migration," van Ooijen wrote.
Overall, he identified these common problems:
- Interpretation differences of the DICOM standard between vendors
- Limited availability of the data of the old PACS due to off-line storage on old media
- Issues from the test migration that must be solved before starting the migration
- Incomplete or incorrect transfer of updates performed on the database of the old PACS when performing a transfer of the data
- Reporting and tracking of issues, which is particularly important during the test migration and migration to determine the status of the project
He recommends that responsibility for the issues must be clear, and regular meetings with all stakeholders, including representatives from both vendors, should take place.



















