Philips Healthcare parent Royal Philips has introduced 3D ultrasound-based technology to monitor abdominal aortic aneurysms (AAAs).
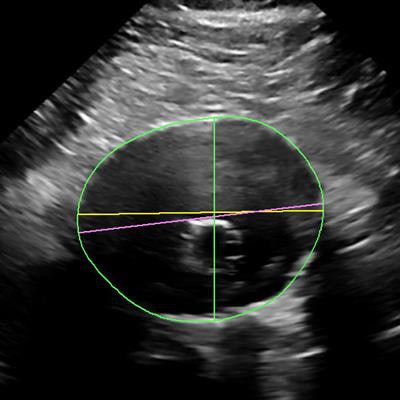
The company's AAA model is designed to assist clinicians by providing key measurements, including the partial volume and centerline of aneurysms.
The AAA model has received the CE Mark in Europe, and the U.S. Food and Drug Administration has cleared it for sale in the U.S. The model is making its debut at the virtual Leipzig Interventional Course (LINC), which is currently underway.
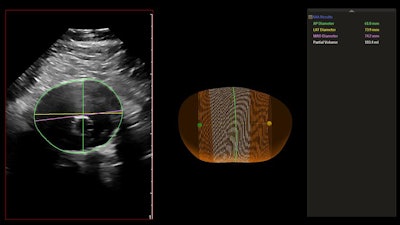 The AAA model provides measurements including the maximum anterior-to-posterior diameter and partial volume of the aneurysm, while also indicating the centerline. Image courtesy of Philips Healthcare.
The AAA model provides measurements including the maximum anterior-to-posterior diameter and partial volume of the aneurysm, while also indicating the centerline. Image courtesy of Philips Healthcare.