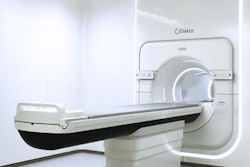
Radiation oncology vendor Elekta has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its image-guided brachytherapy applicator for treating advanced gynecological cancers.
The device, called Venezia, gives clinicians better treatment access to tumors in the cervix, parametrium, vagina, and perineum. It received European CE Mark approval in November 2016, according to the firm.