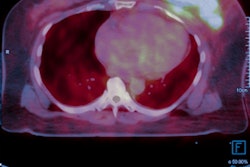
Danish PET technology developer MedTrace said that the first U.S. patient has been scanned in a phase III clinical trial involving its point-of-care manufacturing and dosing system for oxygen-15 (O-15) water.
The clinical trial, called Rapid Water Flow, is a prospective, open-label, multicenter study evaluating the diagnostic accuracy and safety of the firm's radioactive water PET imaging technique in detecting coronary artery disease (CAD). It's also the first phase III trial for a PET myocardial perfusion imaging agent that utilizes blood flow quantification as part of the primary endpoint, according to MedTrace.
The company plans to enroll 182 participants for the trial at 10 locations in the U.S. and Europe. All participants will receive two doses of O-15 water as part of a single PET imaging session. One dose will be provided at rest, while the other one will be given during pharmacological stress with adenosine, MedTrace said. The vendor plans to wrap up the trial in 2024.
MedTrace's PC MT-100 system is designed to enable hospitals to produce the O-15 water injection at the point of care without relying on third-party suppliers for radiopharmaceuticals. Its goal is to develop an automated method that combines production, dosing, injection, and quantification of O-15 water for myocardial perfusion imaging.