Clarity Pharmaceuticals, the Australian-based radiopharmaceutical developer, has completed patient recruitment in the dosimetry phase of a study of its theranostic agent with copper-based radioisotopes for treating metastatic castration-resistant prostate cancer.
Clarity is investigating targeted copper theranostics using the company's SAR-bisPSMA radiopharmaceuticals, which target prostate-specific membrane antigen (PSMA) expressed by prostate tumors. Tumors can first be detected with PET using a diagnostic dose of copper-64 (Cu-64) SAR-bisPSMA; if cancer is found to be present, a version of SAR-bisPSMA with a diagnostic payload of copper-67 (Cu-67) can be injected.
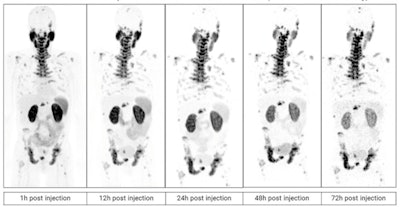 PET scans in a patient with metastatic castration-resistant prostate cancer imaged over multiple time points between one and 72 hours after administration of Cu-64 SAR-bisPSMA. Image courtesy of Clarity Pharmaceuticals.
PET scans in a patient with metastatic castration-resistant prostate cancer imaged over multiple time points between one and 72 hours after administration of Cu-64 SAR-bisPSMA. Image courtesy of Clarity Pharmaceuticals.The trial underway is a multicenter single-arm dose escalation trial set up to determine the safety and efficacy of Cu-67 SAR-bisPSMA as a therapy. The trial is planned to include up to 44 patients in the U.S.
The company reported that the PET images that have been acquired so far look "promising," confirming that SAR-bisPSMA demonstrates high tumor targeting and retention abilities while washing out in other tissues. The company plans to compare its technology with standard bone scans for determining whether prostate cancer has metastasized to the bone.