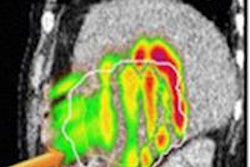
Radiopharmaceutical firm IBA Molecular has submitted documentation to the U.S. Food and Drug Administration (FDA) for a compact proton therapy gantry.
The submission includes full documentation from recently completed in-factory performance and safety tests of its compact gantry beam line. The compact gantry beam line has already been shipped to Willis-Knighton Cancer Center (WKCC) as an element of the first Proteus One system.
Three Proteus One systems have been ordered to date, with patient treatment expected to start in 2014.