The development of AI and deep learning-based software provides automated, time-saving solutions that significantly aid neuroradiologists in catching Alzheimer's disease early, staging it accurately and monitoring the evolution and treatment response, according to prizewinning researchers.
These advances should rapidly find their way into routine clinical practice because they will improve the quality of life in Alzheimer patients, noted Dr. Alexandru Fulga, a radiology resident at Clinica Sanador Victoriei in Bucharest, Romania, and colleagues. To reap the benefits, however, radiologists must become more familiar with basic anatomical, pathophysiological, and clinical notions of Alzheimer's disease, the neuroimaging modalities, and specific findings of this condition, and they also need to know about deep learning-based algorithms with applications in earlier diagnosis, development of prediction models, and treatment research.
"With the emergence of AI and deep-learning algorithms, numerous solutions are available for semi-automated and automated brain segmentation and morphometry," they pointed out in an ECR 2024 e-poster that received a magna cum laude award. "There is plenty of subscription-based or free software (FreeSurfer, volBrain) based on complex deep-learning pipelines which in a matter of minutes can automatically segment all brain structures and calculate their volumes, percentages of total intracranial volume and even compare them with normal values within the same sex and age group."
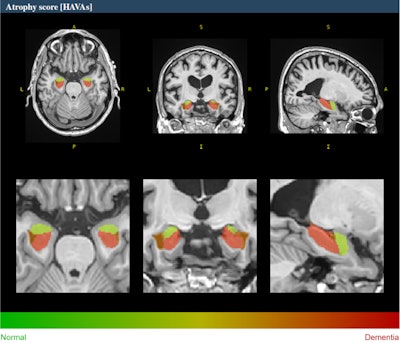 Example of HAVA score using the volBrain platform for a 70-year-old male with late mild cognitive impairment. All figures courtesy of Dr. Alexandru Fulga et al and presented at ECR 2024.
Example of HAVA score using the volBrain platform for a 70-year-old male with late mild cognitive impairment. All figures courtesy of Dr. Alexandru Fulga et al and presented at ECR 2024.
This software requires the input of non-enhanced 3D MP-RAGE T1-weighted series converted into a nii.gz format; a series of preprocessing steps are applied to place the images in the same intensity and geometrical space as the template libraries used to train the pipeline and then, based on those templates, the segmentation and volume estimation is performed, resulting in a highly detailed report, they continued.
Complex hippocampal anatomical atlases can be used to train neural networks, making it possible to automatically perform the subfield segmentation and morphometry of the hippocampus using software like Freesurfer or volBrain.
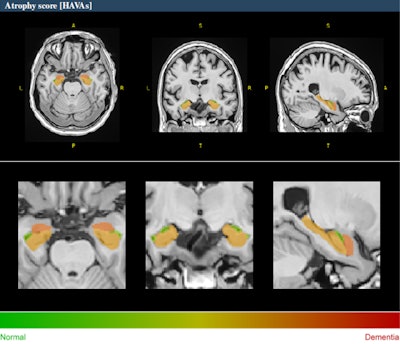 Example of HAVA score using the volBrain platform for a 70-year-old male with early mild cognitive impairment.
Example of HAVA score using the volBrain platform for a 70-year-old male with early mild cognitive impairment.
A novel deep-learning-based tool for Alzheimer's detection and mild cognitive impairment prognostic has been developed, relying on estimated lifespan trajectories of brain structures, the authors explained. Hippocampal-amygdalo-ventricular Alzheimer scores (HAVAs) are based on lifespan models of normal population and Alzheimer's patients, which after validation showed a great capability of detecting patients with Alzheimer's compared with control subjects and specific discrimination between progressive and stable mild cognitive impairment.
The pipeline is freely available and integrated into the volBrain online platform (see: volBrain: An Online MRI Brain Volumetry System, Front Neuroinform, 27 July 2016). It generates volumes of the hippocampus, amygdala, and inferior lateral ventricle, graphics showing where the patient's values stand between the estimated lifespan trajectories of healthy and Alzheimer's patients and it also calculates a probability score based on the three regions analyzed. The method has shown considerable accuracy both in the diagnostic task and the prognostic task.
Neuroimaging plays a key role in diagnosing and monitoring of Alzheimer's because it depicts neuropathological, structural, and functional changes, even from the early stages, they added. For instance, PET/CT can depict Alzheimer's biomarkers such as hypometabolism, beta-amyloid and NFT deposition in specific sites and patterns using radioactive tracers: Fluorodeoxyglucose-18 (FDG-PET), F-18 flortaucipir, F-18 SNFT-1 (tau targeting radiotracers) and Florbetapir (F-18), C-11 PiB (radiotracers binding to beta-amyloid).
Neurodegenerative changes translate into hypometabolism in affected areas -- seen as "cold zones" on FDG-PET -- but they are not specific for Alzheimer's, so efforts are being made to optimize this modality using technological innovations. One application is the multimodal fusion of MRI and FDG-PET images to get a more detailed insight regarding structural and metabolic changes, enabling a better depiction of specific patterns and disease evolution.
"Sometimes visual and ROI-based measurements of FDG-18 uptake can be difficult, especially at the early stages of the disease or when there is the need to distinguish the substages," the researchers noted. "Recently there has been an increase in broad network-based models which after training on large databases showed promising results in automatically detecting hypometabolic changes, patterns, and improving the accuracy of Alzheimer's detection and classification."
The pathological hallmark of Alzheimer's is the deposition of neurotoxic proteins: beta-amyloid and neurofibrillary tangles. Specific radiotracers have been developed that bind precisely to those proteins, offering a non-invasive visual representation of the disease, but amyloid plaques can accumulate years prior to the diagnosis, making it hard to assess the progression of accumulation because it can be already saturated at the time of diagnosis, they explained. Tau PET scans, on the other hand, can reflect directly the pathological deposition and show a close relationship with cognitive function and disease progression, and this makes it a very appealing imaging method, especially for research purposes.
"Innovative convolutional neural network-based algorithms are being developed in order to be applied to tau-scans to improve the detection of tau deposits, discover new patterns of disease, with applications in early diagnosis, accurate discrimination between overlapping stages and treatment response assessment," the authors wrote.
Functional MRI (fMRI) offered tools to study the functional neuronal changes in Alzheimer's, revealing new perspectives regarding alterations between the MTL structures and their complex whole-brain networks. fMRI imaging is based on endogenous blood oxygen level-dependent (BOLD) contrast, depicting neuronal activity during various active or resting state cognitive tasks. Multiple studies have shown a decrease or absence of medial temporal lobe (MTL) activation in Alzheimer's patients in response to various novel stimuli and an elevated MTL activity in response to positive stimuli, contrary to healthy controls, they added.
Several studies have also shown that not only the MTL structures are affected in the early stages but also the complex neural pathways interconnecting the hippocampus with the whole brain. "There is also evidence of an increase in fMRI activation in the neocortex of the frontal and parietal regions which are less affected by the disease, most likely as a compensation mechanism. Now deep learning-based applications for fMRI studies are being developed, showing promising results, to obtain a reliable tool that is able to clearly define and detect functional abnormalities from the earliest signs of Alzheimer's."
The co-authors of the ECR presentation were Dr. Alexandra Catalina Ciurescu, Miruna Calinescu, and Prof. Bogdan Popa. You can access the full exhibit on the EPOS website of the European Society of Radiology.