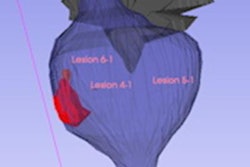
Lucida Medical has secured Lucida Medical has secured MDR Class IIb CE certification for its AI-based prostate cancer diagnosis software for MRI. New research on the AI tool was presented recently at the International Cancer Imaging Society meeting, and it will be available in Europe in early 2024.
The software, called Pi, improves accuracy and reduces the time and cost of prostate cancer diagnosis, according to the firm. It also improves the radiologist "miss rate" from MRI findings for prostate cancer diagnosis and reduces the number of patients who do not have significant cancer but are referred for potentially avoidable biopsies.
To get the full story, CEO Dr. Antony Rix, PhD, urges readers to refer to two key abstracts from the 22nd International Cancer Imaging Society Meeting and Annual Teaching Course at https://doi.org/10.1186/s40644-023-00611-5.
"Abstract P9 describes the ground-breaking multi-centre, multi-vendor external validation of the Lucida Medical Pi software," he told AuntMinnieEurope.com. "Abstract P10 presents tantalising research that indicates that by combining AI, clinical and lab test findings, we can significantly improve performance compared to AI or clinical reporting alone."
Lucida plans to demonstrate Pi at the upcoming RSNA meeting in Chicago in November. The software will be commercially available in the U.K. and the European Union in the first quarter of 2024, Lucida said.