Automated daily quality control (QC) implementation has helped to transform mammography safety and efficiency through the early detection of equipment issues, researchers from Sweden have reported.
Introducing new QC procedures over the past five years has led to significant benefits, according to Tanny Visanuyanont, from the Department of Medical Physics at Ryhov County Hospital in Jönkoping, and colleagues.
These advantages include the early detection of detector errors with precise localization, leading to timely recalibration or detector replacement before clinical use. Another bonus has been the identification of detector changes over time, enabling proactive maintenance and equipment management. Financial savings have also occurred through investment in detector insurance for the entire mammography department, based on the insights gained from the QC process, they pointed out in an e-poster presentation at EuroSafe 2025.
How the system works
Breast screening programs operate under high pressure, handling numerous patients, mostly healthy women, the authors explained. Periodic quality checks should not take long to perform, especially in a stressful clinical environment where downtime should be minimized.
While internal detector calibration occurs at startup, an external, quick, and reliable daily QC procedure is crucial to ensure equipment performance before screening, they continued.
In Jönkoping, daily QC is performed using a 40 mm plexiglass block covering the detector area. The unprocessed image is automatically analyzed by Dosestat QC software from Viximed AB. This software examines exposure parameters, mean pixel value (MV), signal-to-noise ratio (SNR), and standard deviation (SD), and the results and feedback appear in the user interface almost immediately. All data are preserved on the medical physicist interface.
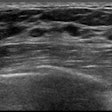
 Daily quality control of mammography equipment with a 40 cm plexiglass block covering the entire detector. Photo courtesy of Tanny Visanuyanont et al and presented at EuroSafe 2025.
Daily quality control of mammography equipment with a 40 cm plexiglass block covering the entire detector. Photo courtesy of Tanny Visanuyanont et al and presented at EuroSafe 2025.
For the analysis, the software divides the image into five sections: top-left, top-right, bottom-left, bottom-right, and the breast area, which covers the most essential part of automatic exposure control. The breast area section is presented separately.
Each region of interest (ROI) of approximately 1 cm² has a fixed position. The software identifies each pixel value and calculates the ROI's mean MV, SD, SNR, and percentage deviations from its own section for MV (Diff MV) and SNR (Diff SNR).
The image analysis is enhanced by implementing fixed ROI positions and comparing individual pixel values to the mean ROI value, the researchers stated. This approach allows for more precise detection of pixel errors and deviations.
"SNR was previously found to vary greatly between the breast area and the area outside the nipple due to heel effect and lag or ghosting from previous screening images," they wrote. "Dividing the image analysis into sections improved trend analysis and baseline setting."
The SNR visualization on the user interface was redesigned using a grayscale characterization that turns out to be specific to each mammography equipment. High SNR values are displayed in dark gray, while low SNR values appear in light gray. This new visualization replaces the previous homogeneous image display, they added.
"The user interface now features an interactive element, allowing users to click on the image and view separate sections of small ROIs with calculated values. If one or more ROI turn pink, this indicates strongly deviating pixels or dead pixels. Red dots in the ROI show the exact location of possible dead pixels," the authors noted.
Improvements on the medical physicist interface have been made to exposure parameter tuning traceability by displaying the history of changed tolerances. This feature enhances the ability to track and analyze equipment performance over time, they said.
Overall, the procedures have enabled timely preventive actions and maintenance, boosted staff confidence in equipment readiness, provided immediate alerts for QC failures via email to medical physicists, and facilitated quick decision-making on equipment usability, the authors noted.
You can read the full EuroSafe 2025 poster here. The co-authors of this exhibit were Emanuel Hillberg and Tomas Moberg, co-founders of Viximed AB, Uppsala.