Liver cirrhosis results in cardiac changes that are more extensive than previously established, according to a study published on 5 June in European Radiology.
Despite preoperative screening, cardiac-associated death remains a leading cause of post-transplant mortality in cirrhosis patients, explained Dr. Jennifer Erley and colleagues in the Department of Diagnostic and Interventional Radiology and Nuclear Medicine at the University Medical Center Hamburg-Eppendorf, Germany. While patients with hepatic pathologies have a high cardiovascular risk profile and are likely to suffer from cardiovascular disease, such as coronary artery disease, recent research shows a connection between liver dysfunction and cardiac damage, termed cirrhotic cardiomyopathy, they explained.
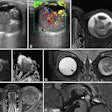
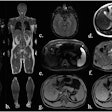
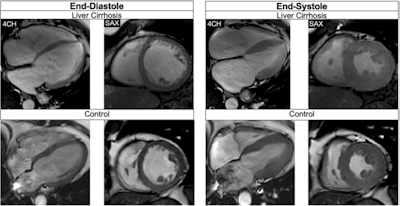 Balanced steady-state free precession cine-images at end-diastole and end-systole (4-chamber (4CH) and midventricular short axis (SAX) views) of a 51-year-old male patient with liver cirrhosis due to primary sclerosing cholangitis (MELD score: 16 points, Child-Pugh class B) and of a 53-year-old male control. The patient showed a higher LVEDVi (108.3 versus 71.8 mL/m2)/RVEDVi (112.1 versus 67.5 mL/m2), a higher LVEF (73.0 versus 64.0%)/RVEF (60.5 versus 57.0%), and increased biventricular strain (LVGLS: −26.0%, LVGRS: 39.9%, LVGCS: −20.4%, RVGLS: −22.9%, RVGRS: 12.3%, RVGCS: −8.0%) than the age-matched control (LVGLS: −14.0%, LVGRS: 19.0%, LVGCS: −17.0%, RVGLS: −22.8%, RVGRS: 13.3%, RVGCS; −7.3%). Furthermore, the patient showed an elevated ECV (32.0%) compared to the control (22.4%).All figures courtesy of Dr. Jennifer Erley et al and European Radiology.
Balanced steady-state free precession cine-images at end-diastole and end-systole (4-chamber (4CH) and midventricular short axis (SAX) views) of a 51-year-old male patient with liver cirrhosis due to primary sclerosing cholangitis (MELD score: 16 points, Child-Pugh class B) and of a 53-year-old male control. The patient showed a higher LVEDVi (108.3 versus 71.8 mL/m2)/RVEDVi (112.1 versus 67.5 mL/m2), a higher LVEF (73.0 versus 64.0%)/RVEF (60.5 versus 57.0%), and increased biventricular strain (LVGLS: −26.0%, LVGRS: 39.9%, LVGCS: −20.4%, RVGLS: −22.9%, RVGRS: 12.3%, RVGCS: −8.0%) than the age-matched control (LVGLS: −14.0%, LVGRS: 19.0%, LVGCS: −17.0%, RVGLS: −22.8%, RVGRS: 13.3%, RVGCS; −7.3%). Furthermore, the patient showed an elevated ECV (32.0%) compared to the control (22.4%).All figures courtesy of Dr. Jennifer Erley et al and European Radiology.
The effects of liver cirrhosis on cardiac structure, function, tissue characteristics, and perfusion have not been comprehensively studied, so the authors set out to investigate the impact of cirrhosis on cardiac changes using cardiovascular MRI.
Cirrhotic cardiomyopathy is defined as a combination of systolic and diastolic dysfunction, according to the criteria published in 2019 by the Cirrhotic Cardiomyopathy Consortium. The few studies that have used cardiac MRI to investigate liver cirrhosis have primarily focused on the left ventricular (LV), which in cirrhotic cardiomyopathy partly define the criteria for systolic dysfunction with an LV ejection fraction (EF) of 50% or lower, along with a global longitudinal strain (GLS) of higher than -18%.
The research team hypothesized that pretransplant patients with cirrhosis would show decreased biventricular function, as well as changes in ventricular and atrial structural MRI parameters and tissue characteristics compared with controls.
In addition, they wanted to explore the relationship between disease severity and those parameters, hypothesizing that myocardial ischemia would be prevalent in subjects with liver cirrhosis due to their high cardiovascular risk.
Fifty liver cirrhosis patients awaiting transplants who were regularly visiting the liver transplantation outpatient clinic of the Hamburg facility received a 3-tesla MRI exam and participated in the study from July 2022 to February 2024. The control group comprised 25 age- and sex-matched healthy controls who had also received contrast-enhanced CMR without stress perfusion imaging for other causes. No participants had a known cardiac disease or reported a previous cardiac intervention.
Left and right ventricular and atrial volumes and functions were analyzed, including EF and feature tracking strain analysis. T1/T2 relaxation times and extracellular volume (ECV) were determined.
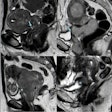
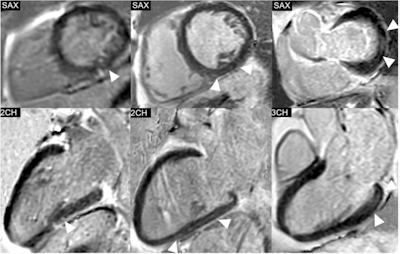 Phase-sensitive inversion recovery (PSIR) images of the left ventricle (LV) in short-axis (SAX) views and the corresponding 2-chamber (2CH) or 3-chamber (3CH) views, showing the distribution of non-ischemic late gadolinium enhancement (LGE) in three exemplary study patients. Left: Punctiform subepicardial LGE of the midventricular inferior LV in a 63-year-old male patient with cirrhosis due to primary sclerosing cholangitis (MELD score: 18 points, Child-Pugh class B). Middle: Linear mid-myocardial LGE of the inferior LV in a 54-year-old male liver cirrhosis patient with chronic Hepatitis C virus infection (MELD score: 15 points, Child-Pugh class B). Right: Linear mid-myocardial LGE of the lateral basal LV in a 57-year-old male liver cirrhosis patient with chronic Hepatitis C virus infection (MELD score: 6 points, Child-Pugh class B).
Phase-sensitive inversion recovery (PSIR) images of the left ventricle (LV) in short-axis (SAX) views and the corresponding 2-chamber (2CH) or 3-chamber (3CH) views, showing the distribution of non-ischemic late gadolinium enhancement (LGE) in three exemplary study patients. Left: Punctiform subepicardial LGE of the midventricular inferior LV in a 63-year-old male patient with cirrhosis due to primary sclerosing cholangitis (MELD score: 18 points, Child-Pugh class B). Middle: Linear mid-myocardial LGE of the inferior LV in a 54-year-old male liver cirrhosis patient with chronic Hepatitis C virus infection (MELD score: 15 points, Child-Pugh class B). Right: Linear mid-myocardial LGE of the lateral basal LV in a 57-year-old male liver cirrhosis patient with chronic Hepatitis C virus infection (MELD score: 6 points, Child-Pugh class B).
Of all assessed cardiovascular risk factors, only the association between diabetes and right ventricular global circumferential strain (RVGCS) was significant in the patients. However, 36% of the patients exhibited valvular diseases on MRI: mitral valve insufficiency (10%), tricuspid valve insufficiency (TI) (6%), mitral and TI (10%), aortic valve insufficiency (6%), aortic and TI (2%), and aortic and mitral valve insufficiency (2%).
Furthermore, liver cirrhosis patients showed a higher LV and RV function compared with controls. None of the patients who received stress testing demonstrated a myocardial perfusion deficit; 20% showed nonischemic late gadolinium enhancement.
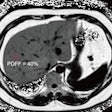
Higher Child-Pugh class and model of end-stage liver disease (MELD) score were associated with an increase in ECV (respectively 4.8%, p = 0.003; 0.5%, p < 0.001), but not with changes in structural and functional MRI parameters.
The results from the study showed that liver cirrhosis does result in cardiac changes that can be assessed on MR, predominantly cardiac dilatation and increased biventricular function. Myocardial fibrosis and elevated extracellular volume were associated with more severe disease, as indicated by the Child-Pugh class and MELD score.
Read the full study here.