Developing a strong understanding of the physical properties and pros and cons of radiotracers is crucial to optimize the clinical applications of SPECT and PET, but results from comparative studies can be difficult to translate into clinical practice and rigorous cost-effectiveness analysis is required, researchers from a leading London facility reported at ECR 2020.
Advances in nuclear medicine mean there is great potential to improve diagnostic accuracies in detecting osseous metastasis, noted Dr. Oi Yean Wong, radiology specialty trainee at the Royal Free London NHS Foundation Trust, U.K., and colleagues in an e-poster presentation.
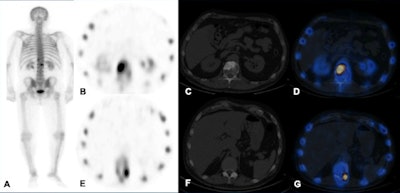 Technetium-99m MDP SPECT/CT examination. A: Whole-body image shows increased tracer uptake at T12 and L1 vertebrae in a patient with breast cancer. B-D: Increased tracer uptake in the sclerotic lesion in the L1 vertebra is in keeping with bone metastasis. E-G: Increased tracer uptake on the left side of T12 vertebra localizes to a facet joint (degenerative disease). All images courtesy of Dr. Oi Yean Wong and Dr. Gopinath Gnanasegaran.
Technetium-99m MDP SPECT/CT examination. A: Whole-body image shows increased tracer uptake at T12 and L1 vertebrae in a patient with breast cancer. B-D: Increased tracer uptake in the sclerotic lesion in the L1 vertebra is in keeping with bone metastasis. E-G: Increased tracer uptake on the left side of T12 vertebra localizes to a facet joint (degenerative disease). All images courtesy of Dr. Oi Yean Wong and Dr. Gopinath Gnanasegaran."Skeletal metastasis is often a poor prognostic indicator and presents late when devastating complications such as fractures and spinal cord compression occur. Other significant complications include pain, hypercalcemia, and bone marrow suppression," they explained. "Therefore, it is important to detect skeletal involvement early and to determine whether patients respond to treatment."
Traditional bone scintigraphy and cross-sectional imaging can be useful, but they are limited in their sensitivity and specificity at detecting bone metastases and at monitoring treatment response. It can be challenging to differentiate active and inactive disease at the suspected sites of bone metastases, but detection of skeletal metastases has improved with the development and application of various radiotracers along with hybrid techniques such as SPECT-CT and PET-CT, according to the authors.
SPECT comes under scrutiny
Like planar scintigraphy, SPECT uses gamma rays. Both these modalities are highly sensitive, widely available, and relatively cheap. Compared with planar scintigraphy, which has poor spatial resolution (1 cm), SPECT-CT can more accurately localize metabolically active foci and characterize them, reducing equivocal reports and allows reliable identification of degenerative disease, often difficult to be distinguished from bone metastasis on other modalities, they stated.
Technetium-99m (Tc-99m) MDP is a bone-specific SPECT and planar scintigraphy tracer. Increased uptake occurs in areas of osteoblastic reparative activity. The main pitfalls are the following:
- Reduced sensitivity to metastases isolated to the bone marrow, as these are usually osteolytic
- Flare response (increased radiotracer uptake that occurs early in the course of treatment) causes diagnostic confusion.
- Assessment of disease progression is limited to emergence of new lesions only. SPECT quantitation techniques are evolving and therefore cannot assess the progression/regression of pre-existing lesions accurately.
What to know about PET tracers
PET has better spatial resolution compared to SPECT, the authors continued. F-18 sodium fluoride (F-18 NaF), F-18 FDG, F-18 choline, gallium-68 (Ga-88) PSMA, and F-18 PSMA are bone-specific and nonspecific PET tracers.
F-18 NaF is a bone-specific tracer. Uptake of F-18 NaF onto hydroxyapatite, the main inorganic component of bone, correlates with bone metabolism and vascularity. The main advantages are the following:
- Better pharmacokinetic characteristics
- High quality images because of high bone to soft tissue activity ratio
- Relatively short imaging time
- Provides quantitative assessment of bone metabolism
- Increased uptake in osteoblastic and mixed lesions that may not manifest as sclerosis on CT
On the flip side, high uptake in areas of elevated osteoblastic activity can also be present in some benign processes, detection of early bone marrow metastases is unclear, small predominant lytic metastases are more likely to be missed, and flare phenomenon results in difficulty assessing treatment response at the early stage, Wong and colleagues added.
F-18 FDG is a nonspecific PET tracer and an analogue of glucose, which is metabolized similarly to glucose. It targets tumor cells that are metabolically active. It can detect both osseous and soft-tissue metastases, and it is a direct tumor-imaging agent, as the tumors utilize the glucose-analogue molecules. In contrast to SPECT, F-18 FDG has high sensitivity for both lytic and marrow metastases. It is useful in both restaging and assessment of treatment response.
However, F-18 FDG is of limited use in detection of sclerotic metastases. Diffuse uptake in marrow is seen after chemotherapy or granulocyte-colony stimulating factor, potentially causing false positives, they pointed out.
F-18 choline tracers are taken into cells by choline transporters. They are subsequently phosphorylated by choline kinase, which is overexpressed in tumors, and finally the tracers are incorporated into cell membranes. They can identify skeletal, marrow-based, and extraskeletal disease, and they have high sensitivity and specificity, for instance compared with a bone scan, and can be useful in assessing treatment response and restaging, the authors wrote.
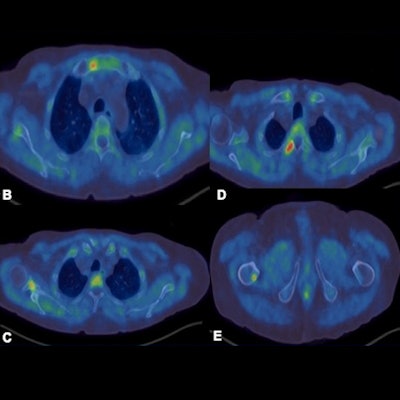
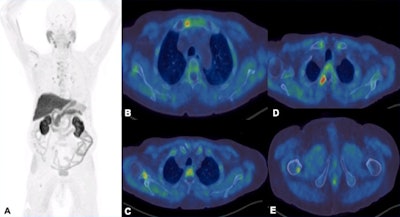 F-18 choline PET-CT examination. A: MIP images show increased tracer uptake in the mediastinum, right axilla and right femur in a patient with prostate cancer. B-E: There is focal increased tracer uptake with the sclerotic lesions in the sternum, right scapula, right transverse process of T3 vertebra, right femur on the fused transaxial PET-CT. Low grade tracer uptake within the mediastinal and hilar nodes are suggestive of reactive/inflammatory changes.
F-18 choline PET-CT examination. A: MIP images show increased tracer uptake in the mediastinum, right axilla and right femur in a patient with prostate cancer. B-E: There is focal increased tracer uptake with the sclerotic lesions in the sternum, right scapula, right transverse process of T3 vertebra, right femur on the fused transaxial PET-CT. Low grade tracer uptake within the mediastinal and hilar nodes are suggestive of reactive/inflammatory changes.However, the sensitivity of the test is influenced by prostate-specific antigen (PSA) levels, and sensitivity increases with higher PSA levels. Also, sensitivity -- and therefore detection rate -- of microcarcinoma is limited by partial volume effect, negative scans do not rule out metastasis, and uptake in densely sclerotic bone lesions is inconsistent, especially after therapy. False positives may occur in recent traumas and fractures.
Prostate-specific membrane antigen (PSMA) tracers are superior to choline tracers in detecting prostate malignancy at low PSA levels, and they can detect both osseous and extra-osseous metastasis, the authors wrote. Also, they have a higher signal-to-noise ratio compared with F-18 choline, and they outperform bone scans in detection of affected bone regions and assessment of overall bone disease volume. Furthermore, they have higher sensitivity, specificity, and overall accuracy in detecting skeletal metastases compared with whole-body MRI.
The main pitfalls are the limited detection of micrometastases, resulting in false negatives, and the false positives in osteoarthritis, degenerative changes, fractures, Paget's disease, hemangiomas, etc., they added.
What the evidence says
Recent studies suggest F-18 NaF PET-CT is superior to Tc-99m MDP planar scintigraphy and SPECT, but comparable to diffusion-weighted MRI, in the detection of bone metastases in prostate cancer patients, the authors wrote.
No significant difference exists between radionuclide studies (SPECT-CT, F-18 NaF PET-CT, F-18 choline PET-CT) and whole-body MRI in the detection of bone metastases in prostate cancer patients. Ga-68 PSMA is superior to whole-body-MRI in detecting bone metastases in prostate cancer, while contrast-enhanced PET-MR has higher sensitivity than contrast-enhanced PET-CT in detecting osseous metastases in breast cancer patients, they stated.
"We're currently working on PET tracers and patterns of uptake in benign tumors in a systematic review of literature and some collaborative projects on F-18 NaF imaging in benign and malignant bone disease," senior author Dr. Gopinath Gnanasegaran told AuntMinnieEurope.com.
"My main interest is in developing nuclear medicine educational modules for both conventional and hybrid imaging," said Gnanasegaran, who is a consultant in nuclear medicine at the Royal Free London NHS Foundation Trust and former chair of the Nuclear Medicine and Molecular Imaging Special Interest Group (SIG) of the British Institute of Radiology (BIR). He is now a member of BIR's Leadership and Management SIG.
Editor's note: to view the authors' full e-poster, go to the ESR's EPOS website.