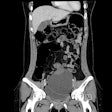
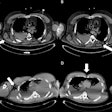
Scientists are moving ahead with research studies using a photon-counting CT unit installed last summer, according to the head of Bayer's contrast media laboratory. The radiology R&D team is now exploring "new frontiers," and a number of preclinical trials using phantoms and animal models have now been performed.
The Naeotom Alpha from Siemens Healthineers was installed at the Berlin facility nine months ago, according to Prof. Hubertus Pietsch, vice president and head of MRI & CT contrast media research at Bayer AG. He explained that an opening in the wall had to be made to accommodate the machine weighing 2.6 tons (see LinkedIn post), and scans on humans are planned soon.
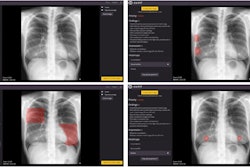
"This revolutionary technology has been likened to the transition from pixelated black-and-white television to high-definition color TV," he explained. "It has the potential to overcome the limitations of traditional CT detectors by providing an unprecedented level of detail, aiding clinical decisions in various fields such as cardiology, oncology, pulmonology, and neurology."
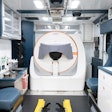
 Prof. Hubertus Pietsch shows the photon-counting CT unit to Liana Gruenberg during the annual Pharma Media event on 20/21 March. Photo courtesy of Bayer AG.
Prof. Hubertus Pietsch shows the photon-counting CT unit to Liana Gruenberg during the annual Pharma Media event on 20/21 March. Photo courtesy of Bayer AG.
An important area of focus of the group is the development of contrast agents designed to minimize the dose while maintaining image quality. For instance, a phase III trial for gadoquatrane, Bayer's next-generation gadolinium-based contrast agent (GBCA), recently entered clinical development for contrast-enhanced MRI. It has a novel tetrameric structure, combining the high stability of a macrocyclic extracellular multipurpose GBCA and high relaxivity with the potential to enable a substantially lower gadolinium dose for patients undergoing a contrast-enhanced MRI, according to Pietsch. The latest preclinical research data were presented at ECR 2024.
There's a long history of collaboration between the two companies in radiology, e.g., the integration of Bayer's CT injectors with Siemens scanners in CT (Medrad Stellant injector and Siemens' Somatom go.Now, plus the Medrad Centargo CT injector with myExam). At ECR 2024, the vendors announced their partnership in the field of fluid delivery systems and adjacent software and services with myExam Contrast; the solution developed with Siemens aims to simplify personalized contrast protocols for patients with data transfer directly into Smart Protocols. Another initiative is the first imaging system interface (ISI) for MRI (MR-Injector coupling via ISI).
"We aim for holistic solutions for doctors and their patients," said Michael McDermott, PhD, head of R&D Innovation for Bayer in Radiology. "Our virtual reality experience depicting a radiology clinic of the future is all about discovering Bayer's digital, integrated, disease-oriented solutions across the entire radiology workflow. With these solutions, we can drive increased efficiency helping radiology staff to handle the growing workload burden caused by increased demand for imaging and rise of chronic diseases from an aging population."
In a press release issued on 21 March, Stefan Oelrich, head of Bayer's Pharmaceuticals Division, stated that its R&D emphasis is on four core therapeutic areas: cardiovascular diseases, oncology, immunology, and neurology and rare diseases. He said the company grew its pipeline with the submission of eight Investigational New Drug applications in 2023 and anticipates that four products will progress to Phase II by year-end.
Earlier this month, Bayer unveiled promising results for acoramidis, an orally administered small molecule, designed as a transthyretin stabilizer for the treatment of transthyretin amyloid cardiomyopathy, he continued. All clinical endpoints were met in a phase III trial and acoramidis is currently under evaluation by the European regulatory authorities for potential marketing approval.
Christoph Koenen, global head of clinical development and operations within pharma R&D at Bayer, emphasized the huge impact of cardiovascular diseases, which remain a leading cause of death globally. With unmet needs persisting in conditions such as stroke and heart failure, Bayer aims to bring novel treatments through its cardiovascular portfolio, he noted.