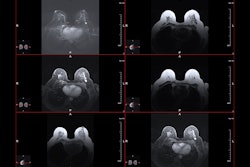
Radiologists must be more active in providing objective and understandable information to women about the diagnostic and prognostic implications of dense breasts, and the value of using other screening methods. That's the view of leading expert Prof. Dr. Christiane Kuhl.
"Women must be enabled to make their own educated decisions and priorities. They have to be informed in an unbiased way about the individual need they have – and the diagnostic options they have – including the respective advantages and side effects of different screening methods," she told AuntMinnieEurope.com, following the statement on 9 March by the U.S. Food and Drug Administration (FDA) to require reporting of breast density information by hospitals.
 Prof. Dr. Christiane Kuhl. Courtesy of Uniklinik RWTH Aachen.
Prof. Dr. Christiane Kuhl. Courtesy of Uniklinik RWTH Aachen.Moreover, they must be informed about the options they have irrespective of whether there are national screening programs, according to Kuhl, who is head of radiology at Aachen University Hospital in Germany and president of the upcoming 104th German Radiology Congress (RöKo).
"For instance, women must be informed about the recommendation to use MRI for screening when they have extremely dense breasts, even if insurance companies do not pay for it, or even if so far there is no national MRI screening implemented for women with dense breasts," she added.
Radiologists are experts in their field and must fill in a leadership role, she continued. "We should issue guidelines that are referred as such," noted Kuhl.
This means the concept of shared decision-making must be adopted also in the field of screening -- just as it has been adopted in the therapeutic arena, according to Kuhl, whose editorial, "What the Future Holds for the Screening, Diagnosis, and Treatment of Breast Cancer," was published in Radiology on 7 February 2023, as part of the U.S. journal's centenary celebrations.
She expressed her disappointment that the pressure to accept women as "grown-ups" had to come from an outside agency like the FDA. "My hope is that the current recommendations of the European Society of Breast Imaging (EUSOBI) -- suggesting that communication and shared decision making are important components of individualized or personalized screening -- are used to change the field from within."
Ongoing education process
The updated FDA regulations are not really a surprise but rather a logical continuation of a process that has been going on for several years, Dr. Ritse Mann told AuntMinnieEurope.com. He thinks they will eventually help to improve education and information within Europe.
 Dr. Ritse Mann, chair of the EUSOBI scientific committee.
Dr. Ritse Mann, chair of the EUSOBI scientific committee."For screening organizations and policy makers, it simply becomes impossible to deny that breast density has an impact on the quality of breast screening," he said. "Staying silent is likely to lead to legal actions against organizations unwilling to share density data with the women being screened."
At the same time, FDA regulations do not generally have a direct impact on European practice, and they are likely only to be "a further argument in a discussion on honesty about -- and ownership of -- medical information that is slowly developing."
In essence, the U.S. authorities increasingly demand that women are informed of medical findings that may affect their risk of developing serious disease, Mann continued.
"To me, this is a logical continuation of the fact that women (and patients in general) are increasingly seen as owners of their own medical data," he said. "This process is also ongoing within Europe, but is somewhat lagging behind."