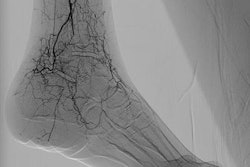
A flat-panel x-ray detector combined with a gamma camera allows simultaneous acquisition of fluoroscopic and nuclear images. Researchers at Utrecht University in the Netherlands constructed a prototype device to demonstrate its feasibility, and they used Monte Carlo simulations to show how refinements could improve its performance. The instrument could provide clinicians with anatomical information not currently available using nuclear imaging alone.
Usually, hybrid images that integrate two different modalities are produced by applying one technique after another. If the patient moves in between the two scans, the component images are misaligned. Capturing both frames at the same time ensures spatial correspondence between them.
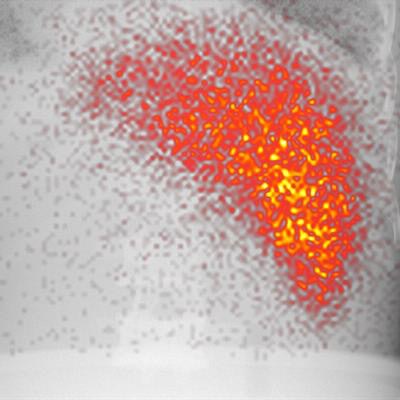
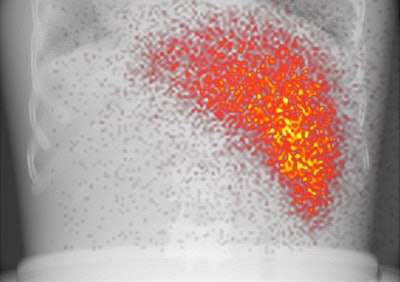 A hybrid image of an anatomical phantom. Activity in the liver is projected on the x-ray background image. Image courtesy of Sandra van der Velden et al.
A hybrid image of an anatomical phantom. Activity in the liver is projected on the x-ray background image. Image courtesy of Sandra van der Velden et al.A previous attempt at combining fluoroscopy and nuclear imaging for simultaneous acquisition saw four gamma cameras with pinhole collimators positioned around the x-ray source on the opposite side of the patient from the x-ray detector. Under that arrangement, the distribution of the gamma emitter had to be reconstructed from four separate projections.
"This was computationally quite demanding, required complicated hardware integration, and resulted in a lower resolution of the nuclear images," noted lead author Sandra van der Velden, a clinical scientist.
Van der Velden and colleagues have shown that useful images can be captured even when a single gamma camera and cone-beam collimator are placed behind the x-ray detector. Composite images acquired in this way are naturally coregistered, meaning that diagnostic and therapeutic radioisotopes can be monitored within the anatomical context provided by fluoroscopy (Radiology, 8 January 2019).
Although the x-ray and gamma detectors are sensitive to different photon energies (30-120 keV for x-rays versus 140 keV for gamma rays), the fact that gamma rays are collected after passing through the x-ray detector in this setup means that system sensitivity is unavoidably compromised. To mitigate this, the researchers used a modified flat panel from which some lead shielding and structural aluminum had been trimmed.
Experiments using radioactive sources in moving phantoms showed that attenuation by the x-ray detector reduced the sensitivity of the gamma component by 45% to 60%, but the hybrid images were still useful and artifact-free. The spatial resolution of the gamma camera was unaffected, and the presence of a radioisotope in its field-of-view also had no effect on the performance of the x-ray detector.
As promising as these results are, the performance of the device could be improved with some realistically achievable modifications. When van der Velden and colleagues modeled the setup using Monte Carlo simulations, they found that a thinner flat-panel x-ray detector with a smaller thickness of aluminum could reduce the impact on system sensitivity to 27% to 35%. This would also allow the gamma camera to be moved closer to the patient, improving the device's spatial resolution.
Benefits in the clinic
The ability to acquire nuclear and fluoroscopic images simultaneously could improve any treatment that involves radioisotopes, but the researchers propose a specific application in radioembolization for liver cancer. In this therapy, the tumor is irradiated and has its blood supply cut off by the injection of radioisotope-filled beads.
Currently, radioembolization is guided by fluoroscopy and preceded by a nuclear imaging procedure that predicts the uptake of the therapeutic radioisotope. These steps are conducted in separate locations, and there can be many days between them.
"We are currently working on a clinical prototype, which is at this moment being constructed," van der Velden said. "We plan to perform a clinical trial with that prototype, to show that radioembolization procedures can be improved. This will open up the possibility to perform it in one day, instead of the current two-step process, which takes one or two weeks."
Conducting the treatment over two visits is inconvenient for the patient and also means that the catheter must be inserted anew each time. By conducting the entire procedure in a single day, the injection location for the imaging and therapeutic isotopes remains constant, making the pretreatment step a more effective predictor of outcome.
Marric Stephens is a freelance science writer based in Bristol, U.K.
© IOP Publishing Limited. Republished with permission from Physics World, a website that helps scientists working in academic and industrial research stay up to date with the latest breakthroughs in physics and interdisciplinary science.