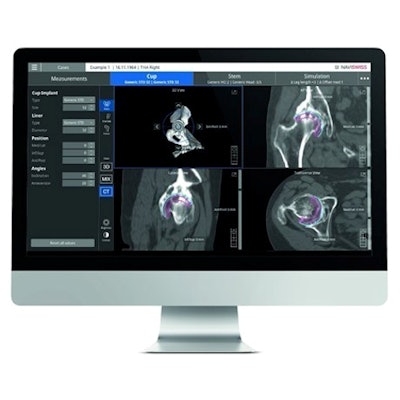
Naviswiss has received clearance from the U.S. Food and Drug Administration (FDA) to market its Naviplan orthopedic planning software in the U.S.
Naviplan is a digital preoperative planning application that allows orthopedic surgeons to perform navigated, CT-based total hip replacement surgery. The company touted the application as potentially improving accuracy and predictability, while providing seamless outcome documentation.
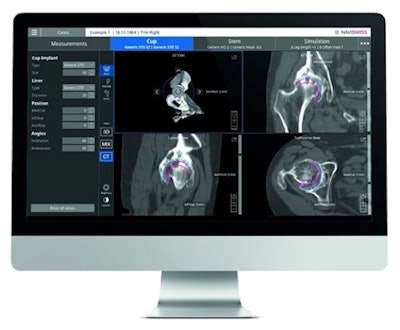 Naviplan is a digital preoperative planning application for orthopedic surgeons. Image courtesy of Naviswiss.
Naviplan is a digital preoperative planning application for orthopedic surgeons. Image courtesy of Naviswiss.Naviswiss is releasing Navigation and Naviplan to orthopedic care centers in the U.S. during the fourth quarter of 2021.