Icometrix has gained approval to market its icobrain aria software in Europe, Israel, Japan, Switzerland, and the U.K.
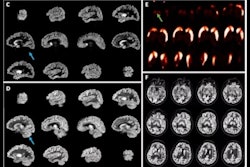
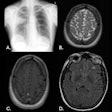
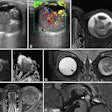
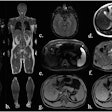
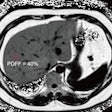
Icobrain aria is designed for automated quantification of amyloid-related imaging abnormalities (ARIA) and severity scoring of ARIA-E (edema/sulcal effusion) and ARIA-H (hemorrhage/superficial siderosis). The software is now certified under European Union (EU) CE Medical Device Regulation (MDR) and otherwise registered in key regions, company officials told AuntMinnieEurope.com.
Icometrix expects that ARIA safety monitoring on regular brain MRI scans will play an important role in the follow-up of patients treated with amyloid beta-directed monoclonal antibodies for Alzheimer’s disease.
"Clinical trials have shown that these treatments can be associated with amyloid-related imaging abnormalities (ARIA),” Prof. Sebastiaan Engelborghs of the Vrije Universiteit Brussel in Belgium and Universitair Ziekenhuis Brussel Hospital said in a company announcement.
Icometrix also noted that it has been recommended that patients receive three brain MRI scans during the first year of treatment to detect and monitor ARIA. The software will aid radiologists and neurologists in assessing ARIA and prescribing anti-amyloid treatments, the company said.
In the U.S., icobrain aria is pending 510(k) approval from the U.S. Food and Drug Administration (FDA), Icometrix said.