For effective implementation of lung cancer screening, start with a program targeting those most at risk and employ pathways minimizing risk of harm, including those due to overdiagnosis, and then expand as unanswered issues are clarified, U.K. specialists have advocated.
A biennial or annual screen starting at 60 years old in those with a risk of lung cancer in excess of 1% per annum, with clear protocols for the management of abnormal findings would fit with ongoing research into the best methods for selection and recruitment, noted Dr. David Baldwin, professor at the Faculty of Medicine and Health Sciences at Nottingham University Hospitals in the U.K.
"One key remaining issue is recruitment of people into screening programs. Lung cancer is much more common in less economically advantaged groups, who also represent a hard-to access group," he explained, adding that his team is now looking closely at the optimal screening intervals, and the U.K. Lung Cancer Screening Trial (UKLS) is applying for funding for a further round of screening, as well as a number of projects looking at ways to recruit people who are at high risk but less likely to say yes to screening.
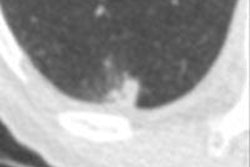
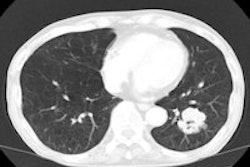
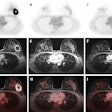
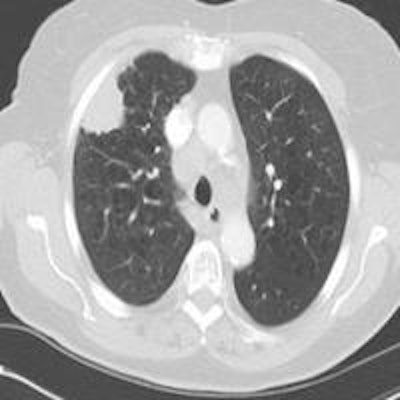
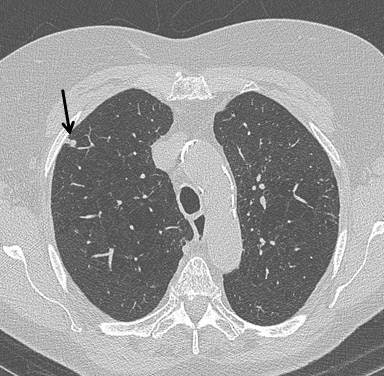 Top: Early stage lung cancer detected by CT. The 10-year survival rate is more than 90%. Bottom: A stage IIIa lung cancer where cure is unlikely. Solid line primary cancer can be seen, and the dotted line shows mediastinal nodes. All images courtesy of Dr. David Baldwin.
Top: Early stage lung cancer detected by CT. The 10-year survival rate is more than 90%. Bottom: A stage IIIa lung cancer where cure is unlikely. Solid line primary cancer can be seen, and the dotted line shows mediastinal nodes. All images courtesy of Dr. David Baldwin.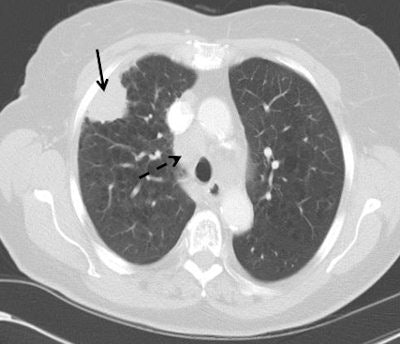
In addition to selection and recruitment, important questions remain about level of harm, optimal clinical pathways, and cost-effectiveness. The recommendations made by the U.S. Preventive Services Task Force (USPSTF) may have gone too far, not by extending screening to the older age group, but by including younger people without a more sophisticated estimate of the risk of lung cancer, according to Baldwin and his co-author Dr. Emma O'Dowd, clinical research fellow in the Division of Public Health and Epidemiology at the University of Nottingham (British Journal of Radiology, 11 November 2014).
More recent publications have suggested that by better selection using a risk prediction model, there might have been more lives saved with reduction in the number needed to screen to 270, and if screening were to have been restricted to the subjects with a 1.25% risk of lung cancer death over five years, this falls further to 166, they stated. Better selection methods are available and validated, and their use may go some way to placate the recently expressed reservations about CT screening from Medicare and Medicaid.
Low dose CT reduces the radiation dose to approximately one-fifth of a typical thoracic CT, and this equates to less than half a person's annual background radiation dose, the authors wrote. Based on modeling, the USPSTF estimated that annual CT screening for lung cancer would cause one radiation-induced lung cancer death for every 22 prevented lung cancer deaths, but further improvements in technology may enrich this figure.
CT detects many benign nodules and masses (about 25 for every cancer detected), they continued. In the National Lung Screening Trial (NLST), 59/26,722 (0.2%) of participants underwent a CT-guided lung biopsy for a benign lesion and seven (0.03%) had a major complication. Clinical management pathways were not prespecified in the NLST, and where data were available, 2.2% of participants underwent PET, thus receiving a much higher radiation dose. The Dutch-Belgian NELSON trial and the UKLS pilot trial both employ low-dose CT follow-up by using volume measurements rather than diameter to detect growth, and this reduces the number of false-positive screens and defines more accurately the need for invasive procedures.
"Optimal screening follow-up pathways are becoming clear and will limit false-positive tests and the harm from further investigation," they noted. "People who are overdiagnosed do not benefit from early diagnosis but are subject to the same harm as others from investigations, treatment, and the psychological impact of being told they have cancer. They may, however, benefit from health interventions that are provided with screening such as smoking cessation advice."
Cost-effectiveness debate
The cost-effectiveness of a program will be strongly influenced by the frequency of screening, duration of screening program, risk profile of the screened population, uptake of screening in hard-to-reach groups and smoking cessation, and the range of reported incremental cost-effectiveness ratios (ICERs) remains considerable, according to Baldwin and O'Dowd.
A U.S. simulation of the annual screening of ever-smokers using national epidemiological and Mayo Clinic CT trial data estimated the ICERs to be $110,000-$160,000 (88,300 to 128,400 euros) per quality adjusted life year (QALY) gained. Also, a U.S. stage-shift model using the Early Lung Cancer Action Program (ELCAP) protocols and outcomes data, Medicare tariffs and national survival rates by stage-produced ICER estimates less than $19,000 (15,257 euros).
The model was re-estimated for annual screening and yielded baseline estimates of $28,000 (22,484 euros) or $47,000 (37,700 euros), depending on whether the model's cancer stage predictions derived from the ELCAP -- or the NLST reported stage shift; costs fell further with smoking cessation to $16,000-$23,000 (13,000-18,500 euros), respectively. The lowest ICER estimated is around $1,464 (1,176 euros) per QALY gained, reported for Israel.
Publication of NLST has generated much international discussion, and the U.S. has gone the furthest in recommending national implementation of screening, they explained.
"The solution will vary according to the individual country's healthcare system and may increase the work of radiologists and radiographers. The efficient use of nodule management algorithms and trained nonradiologist readers in combination with computer-aided detection (CAD) software may mitigate this," the authors believe. "Private healthcare providers are offering CT screening in many countries and the concern is that this is not regulated. The importance of adherence to standards that serve to minimize harm and maximize benefit has been emphasized."
The U.K. Committee on Medical Aspects of Radiation Exposure is due to publish a report soon, further emphasizing the need for an expert approach in the independent sector, but the most important concern with private provision of screening is that it does nothing for the majority of those at risk, as lung cancer is more common in the less advantaged, they wrote.
"Those of us who care for patients with lung cancer want screening to be introduced as soon as possible for all those at risk, but it has to be on the basis that it is cost-effective because of the high total cost," they concluded.