GE HealthCare has revealed plans to launch its macrocyclic, nonionic MRI gadolinium-based contrast agent in "a number of" European countries this year under the name Pixxoscan.
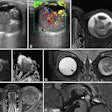
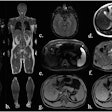
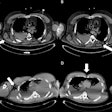
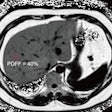
The company said the gadobutrol contrast agent helps with visualizing abnormal structures or lesions as well as differentiating between healthy and pathological tissue. In line with the reference product, Gadovist, Pixxoscan (gadobutrol) is indicated for use in adults, adolescents, and children of all ages for contrast enhancement in cranial and spinal MRI and magnetic resonance angiography. GE added that it is also indicated for whole-body imaging.
GE highlighted gadobutrol's high relaxivity and formulation at twice the concentration of gadolinium ions. It said this reduces injection volume by half compared with other gadolinium-based contrast agents.
Pixxoscan's packaging will also consist of glass vials, ready-assembled plastic prefilled syringes, and larger volume +Pluspak polypropylene bottles.
The company added that the agent will be produced in compliance with current Good Manufacturing Practices (cGMP), with testing being done at its primary and secondary manufacturing sites in Norway. Once approved, it will then be labeled and packed for shipping.
Pixxoscan has been reviewed using a regulatory decentralized procedure with marketing authorization already in place in Austria and pending approval. In France, Pixxoscan will be known as Pixcyclic, according to GE.