International experts have unveiled a set of guidelines they say could help specialists and clinicians who perform F-18 FDG and radiolabeled amino acid PET imaging in pediatric patients affected by brain gliomas.
"For oncological brain imaging, different amino acid PET radiopharmaceuticals have been introduced in the last years," wrote corresponding author Dr. Lars Kurch of University Hospital Leipzig, Germany. The guidelines were presented recently in an article in the European Journal of Nuclear Medicine and Molecular Imaging.
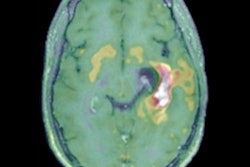
The article proposes guidelines for indication, acquisition, and interpretation of F-18 FDG and radiolabeled amino acid PET imaging in pediatric patients affected by brain gliomas. Brain gliomas are responsible for up to 27% of all malignancies in children ages 14 and younger and up to 10% of those occurring in adolescents between the ages of 15 and 19. Central nervous system neoplasms are the main cause of pediatric cancer-related death, the authors added.
Considering the increasing use of hybrid imaging with PET/CT and PET/MRI in pediatric glioma, new guidelines are necessary that provide proper information about patient selection, preparation and precautions, useful radiopharmaceuticals, radiation exposure, acquisition protocol, and image interpretation, the authors stated.
The guidelines were developed collaboratively by the European Association of Nuclear Medicine and the Society for Nuclear Medicine and Molecular Imaging, together with the European Society for Pediatric Oncology Brain Tumor Group and the Response Assessment in Pediatric Neuro-Oncology working group.